��Ŀ����
2008��5��26�����磬��������ʥ�������д��ݡ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ơ���������в��ϣ��ش��й����⣺
(1)���Ͻ�����е�����ͨ�����з�Ӧ�Ƶõģ�2Al2O3 4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
(2)���ƻ�����õ�ȼ���DZ���(��ѧʽΪC3H8)���������(CH4)������(C4H10)����ͬһϵ�е��л���--����������ϵ���л�������(C5H12)������(C6H14)���������ʡ������������������Ļ�ѧʽ�����ƣ���֪�����к�����̼ԭ�ӵ������Ļ�ѧʽ��________�����������к�������ߵ�������________��
(3)������ȫȼ�����ɶ�����̼��ˮ�����仯ѧ��Ӧ����ʽΪ________________________��
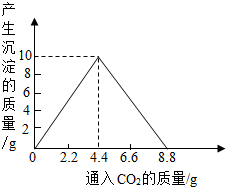
(4)����������ȫȼ�պ������CO2���壬ͨ��һ�����ij���ʯ��ˮ�У������ij�����ͨ��CO2�����������ϵ����ͼ��ʾ����������������������______g�������ԭʯ��ˮ���������ʵ�������[��֪��CaCO3+CO2+H2O��Ca(HCO3)2��Ca(HCO3)2������ˮ]
(1)���Ͻ�����е�����ͨ�����з�Ӧ�Ƶõģ�2Al2O3
 4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��(2)���ƻ�����õ�ȼ���DZ���(��ѧʽΪC3H8)���������(CH4)������(C4H10)����ͬһϵ�е��л���--����������ϵ���л�������(C5H12)������(C6H14)���������ʡ������������������Ļ�ѧʽ�����ƣ���֪�����к�����̼ԭ�ӵ������Ļ�ѧʽ��________�����������к�������ߵ�������________��
(3)������ȫȼ�����ɶ�����̼��ˮ�����仯ѧ��Ӧ����ʽΪ________________________��
(4)����������ȫȼ�պ������CO2���壬ͨ��һ�����ij���ʯ��ˮ�У������ij�����ͨ��CO2�����������ϵ����ͼ��ʾ����������������������______g�������ԭʯ��ˮ���������ʵ�������[��֪��CaCO3+CO2+H2O��Ca(HCO3)2��Ca(HCO3)2������ˮ]

(1)-1
(2)C2H6������(��CH4)
(3)C3H8+5O2 CO2+4H2O
CO2+4H2O
(4)10
(2)C2H6������(��CH4)
(3)C3H8+5O2
 CO2+4H2O
CO2+4H2O(4)10

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
�����Ŀ
 2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺
2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺ ������˺Ż�����½̽������2008��5��26���峿7ʱ53�������ڻ��DZ��������ɹ���½����̽�������������ڵ���������ͼ��ʾ�ǻ��Ǵ��������ɷ�ʾ��ͼ��������ɷ���Ƚϣ�����˵����ȷ���ǣ�������
������˺Ż�����½̽������2008��5��26���峿7ʱ53�������ڻ��DZ��������ɹ���½����̽�������������ڵ���������ͼ��ʾ�ǻ��Ǵ��������ɷ�ʾ��ͼ��������ɷ���Ƚϣ�����˵����ȷ���ǣ�������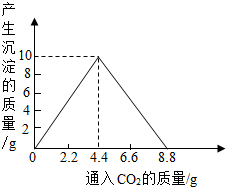 2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺
2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺ 4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
 4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ______��
4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ______��