��Ŀ����
��̼����������ָͨ��һ���ķ���������ҵ�����в�����CO2����������д�������õĹ��պͼ�����
��1������п�ѧ���������CO2�����ߵ����崵�뱥��KHCO3����Һ�У�����KHCO3��Ȼ������KHCO3���ٰ�CO2������ѧ��Ӧ ʹ֮��Ϊ�״���CH3OH����ˮ������ɫ���ɡ�����ļ���������ͼ��
ʹ֮��Ϊ�״���CH3OH����ˮ������ɫ���ɡ�����ļ���������ͼ��
 ʹ֮��Ϊ�״���CH3OH����ˮ������ɫ���ɡ�����ļ���������ͼ��
ʹ֮��Ϊ�״���CH3OH����ˮ������ɫ���ɡ�����ļ���������ͼ��
��ע���ֽ���ڵķ�Ӧ�����Ǽ��ȣ��ϳ����ڵķ�Ӧ����Ϊ300�桢200kPa�ʹ�����
�ٷֽ���ڷ�Ӧ�Ļ�ѧ����ʽΪ____________________________ ��
�ںϳ����ڷ�Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��X��Һ��ѭ�����ã�������___________����д��ѧʽ����
��2��ʵ�������� ����������NaOH��Һ����CO2������ͼ��ͼ����������������δ�������
����������NaOH��Һ����CO2������ͼ��ͼ����������������δ�������
�ٷֽ���ڷ�Ӧ�Ļ�ѧ����ʽΪ____________________________ ��
�ںϳ����ڷ�Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��X��Һ��ѭ�����ã�������___________����д��ѧʽ����
��2��ʵ��������
 ����������NaOH��Һ����CO2������ͼ��ͼ����������������δ�������
����������NaOH��Һ����CO2������ͼ��ͼ����������������δ�������
�ٲ����ڷ�Ӧ�Ļ�ѧ����ʽΪ____________________��
�ڡ���Ӧ���롱������Ӧ�Ļ�ѧ����ʽΪ________________________��
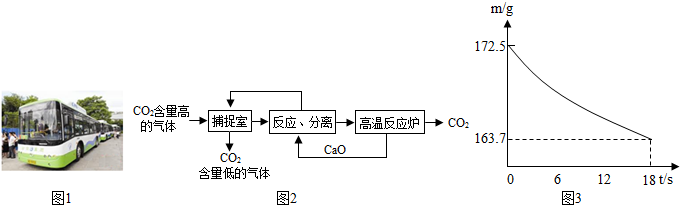
�� �����������У��漰�����������ڼ����______________________����д��ѧʽ����
�����������У��漰�����������ڼ����______________________����д��ѧʽ����
�ڡ���Ӧ���롱������Ӧ�Ļ�ѧ����ʽΪ________________________��
��
 �����������У��漰�����������ڼ����______________________����д��ѧʽ����
�����������У��漰�����������ڼ����______________________����д��ѧʽ����(1)��2KHCO3 K2CO3+H2O+CO2��
K2CO3+H2O+CO2��
��3H2+CO2 CH3OH+H2O
CH3OH+H2O
��K2CO3
(2) ��CO2+2NaOH=Na2CO3+H2O
��CaO+H2O=Ca(OH)2 ��Ca(OH)2+Na2CO3=CaCO3��+2NaOH
�� Ca(OH)2��NaOH
Ca(OH)2��NaOH

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ