��Ŀ����
�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�

��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ�(Ҫ��д���������)
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߡ�
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ�(Ҫ��д���������)
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߡ�
��1��9.8��16.0
��2���⣺�������ᷴӦ��NaOH������Ϊx ����CuSO4��Ӧ��NaOH������Ϊy��
H2SO4 + 2NaOH��Na2SO4 + 2H2O �� CuSO4 + 2NaOH ��Cu(OH)2��+ Na2SO4
98 80 �� 80 �� 98
9.8g x �� y �� 9.8g


x = 8g y =8g
�ʲμӷ�Ӧ��NaOH��Һ��������Ϊ
�����������вμӷ�Ӧ��NaOH��Һ����������160�ˡ�
��2���⣺�������ᷴӦ��NaOH������Ϊx ����CuSO4��Ӧ��NaOH������Ϊy��
H2SO4 + 2NaOH��Na2SO4 + 2H2O �� CuSO4 + 2NaOH ��Cu(OH)2��+ Na2SO4
98 80 �� 80 �� 98
9.8g x �� y �� 9.8g


x = 8g y =8g
�ʲμӷ�Ӧ��NaOH��Һ��������Ϊ

�����������вμӷ�Ӧ��NaOH��Һ����������160�ˡ�
��3��


��ϰ��ϵ�д�
�����Ŀ
�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�
��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߣ����������
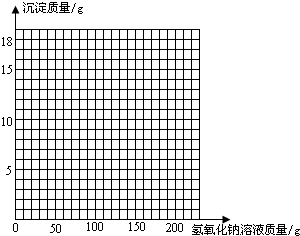
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߣ����������

�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�
��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.45 | 8.6 | 9.8 | 9.8 |
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�