��Ŀ����
̼���ƣ��׳ƴ�������г���������������Ȼ��ƣ�ij�о���ѧϰС����һ������������ʣ�ֻ����Ϊ�Ȼ��ƣ�Ϊ�о�����̽���ô�����Ʒ��̼���Ƶĺ���������������ǵĻ��
��С�����ۡ���
�۵������Ʒ��ֻ����̼��
�۵������Ʒ����̼���ƺ������Ȼ�����ɵĻ����
����Ʋ�����ʵ�顿
�����˼·��������Ʒ��ʯ��ˮ��Ӧ���ɳ���̼��Ƶ����������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ���������
�������裺��ȡ13.25g������Ʒ����������ij���ʯ��ˮ����ֽ��衢���ˡ�ϴ�ӡ�����õ���ɫ����10.00g
�����ݴ��������������ʵ�����ݣ��������Ʒ����Na2CO3������������
������̣�
����ʵ����ۣ�
��ʵ�����ۡ�
����������ɫ�����������ڹ���ϴ�ӣ���������л�������ģ���ɼ�������ʵ��ֵ���ƫС��
��������˼��
��1��ʵ������У����ò��������Ͻ��裬�ò�����Ŀ����
��2��ʵ������У��Գ���ϴ�ӵ�Ŀ���� ��
��С�����ۡ���
�۵������Ʒ��ֻ����̼��
�۵������Ʒ����̼���ƺ������Ȼ�����ɵĻ����
����Ʋ�����ʵ�顿
�����˼·��������Ʒ��ʯ��ˮ��Ӧ���ɳ���̼��Ƶ����������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ���������
�������裺��ȡ13.25g������Ʒ����������ij���ʯ��ˮ����ֽ��衢���ˡ�ϴ�ӡ�����õ���ɫ����10.00g
�����ݴ��������������ʵ�����ݣ��������Ʒ����Na2CO3������������
������̣�
��ʵ�����ۡ�
����������ɫ�����������ڹ���ϴ�ӣ���������л�������ģ���ɼ�������ʵ��ֵ���ƫС��
��������˼��
��1��ʵ������У����ò��������Ͻ��裬�ò�����Ŀ����
��2��ʵ������У��Գ���ϴ�ӵ�Ŀ����
���㣺ʵ��̽�����ʵ���ɳɷ��Լ�����,�εĻ�ѧ����,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
������������̣�������Ʒ��ʯ��ˮ��Ӧ���ɳ���̼��Ƶ����������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
ʵ����ۣ����ݼ��㣬�����ж���Ʒ����ɣ�
��������˼��
��1�����ݲ����������÷�����
��2��ͨ��ϴ�ӣ����Գ�ȥ�����������ʣ�������
ʵ����ۣ����ݼ��㣬�����ж���Ʒ����ɣ�
��������˼��
��1�����ݲ����������÷�����
��2��ͨ��ϴ�ӣ����Գ�ȥ�����������ʣ�������
����⣺��ʵ����ơ��⣺�贿����Ʒ�к�Na2CO3������Ϊx
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 10.00g
=
x=10.6g
������Ʒ��Na2CO3����������Ϊ
��100%=80%
�ɴ˵ó��Ľ����ǣ�������Ʒ����̼���ƺ������Ȼ�����ɵĻ�������Ʒ��̼���Ƶ�����������80%��
��������˼��
��1��ʵ������У�ͨ�����������Ͻ��裬����ʹ��Ӧ��ַ�Ӧ��
��2���ڼ���ʵ������У�ͨ��ϴ�ӿ��Գ�ȥ�����������ʣ�������
�ʴ�Ϊ����ʵ����ơ�������̣��⣺�贿����Ʒ�к�Na2CO3������Ϊx
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 10.00g
=
x=10.6g
������Ʒ��Na2CO3����������Ϊ
��100%=80%
����������Ʒ����̼���ƺ������Ȼ�����ɵĻ�������Ʒ��̼���Ƶ�����������80%��
��������˼����1������ʹ��Ӧ��ַ�Ӧ����2�����Գ�ȥ�����������ʣ��������
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 10.00g
| 106 |
| x |
| 100 |
| 10.00g |
x=10.6g
������Ʒ��Na2CO3����������Ϊ
| 10.6g |
| 13.25g |
�ɴ˵ó��Ľ����ǣ�������Ʒ����̼���ƺ������Ȼ�����ɵĻ�������Ʒ��̼���Ƶ�����������80%��
��������˼��
��1��ʵ������У�ͨ�����������Ͻ��裬����ʹ��Ӧ��ַ�Ӧ��
��2���ڼ���ʵ������У�ͨ��ϴ�ӿ��Գ�ȥ�����������ʣ�������
�ʴ�Ϊ����ʵ����ơ�������̣��⣺�贿����Ʒ�к�Na2CO3������Ϊx
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 10.00g
| 106 |
| x |
| 100 |
| 10.00g |
x=10.6g
������Ʒ��Na2CO3����������Ϊ
| 10.6g |
| 13.25g |
����������Ʒ����̼���ƺ������Ȼ�����ɵĻ�������Ʒ��̼���Ƶ�����������80%��
��������˼����1������ʹ��Ӧ��ַ�Ӧ����2�����Գ�ȥ�����������ʣ��������
���������⽫�����ʵ���л��ؽ���������п��飬ͨ����֤���⣬���������Ѹ���һϵ�е���ʾ��������������⣬�������õ���Ϣ����ַ������룬�����е�֪ʶ������ϵ��Ȼ���������Ƶ����Ӷ��ﵽ��������Ŀ�ģ�

��ϰ��ϵ�д�
�����Ŀ
�������ƣ�NaNO2�������ʳ�μ����ƣ�����ζ�����������Ƹ����������ȣ��ֽܷ�ų��д̼�����ζ�����壬����������ǣ�������
| A��SO2 |
| B��NO2 |
| C��N2 |
| D��NH3 |
�����й�ʵ������������У���ȷ���ǣ�������
| A�����ڿ����о���ȼ�գ���������ɫ���� |
| B�������ڿ����о���ȼ�գ������������� |
| C�������ڴ�����ȼ�գ��������䣬������ɫ����С���� |
| D��һ����̼ȼ���ܲ���ʹ����ʯ��ˮ����ǵ����� |
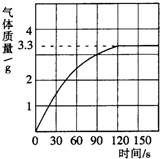 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ��������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������ϡ������뵽10gˮ���У�����CO2������������ͼ��ʾ������֪��ˮ������Ҫ�ɷ���̼��ƺ�������þ�����߶��������ᷴӦ����������þ�����ᷴӦ����������壩
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ��������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������ϡ������뵽10gˮ���У�����CO2������������ͼ��ʾ������֪��ˮ������Ҫ�ɷ���̼��ƺ�������þ�����߶��������ᷴӦ����������þ�����ᷴӦ����������壩