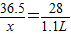��Ŀ����
С��ͬѧ�Ե��ǣ����ǵ���Ҫ�ɷ���̼��ƣ���չ���й�̽�����������£���7.5�˵ĵ��Ƿ��������У������м���������ϡ���ᣬ��ַ�Ӧ���ռ���CO2�������1.1L��CO2���ܶ�Ϊ2g��L���������������̼ȫ�����ɵ����е�̼��������ᷴӦ���������ǵ��������ʲ���ϡ���ᷴӦ����������С��ͬѧ�IJ���ʵ�鱨�棺����μӷ�Ӧ����������Ϊ���ٿˣ�����������£�
�⣺��μӷ�Ӧ����������Ϊx��
CaCO3+ HCl===CaCl2+H2O+CO2��
36.5 28
x 1.1L
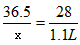
x=3g
�𣺲μӷ�Ӧ����������Ϊ3g��
��1��С���ļ�������г��ֶദ���������г����е���������
�� ���� ��
��2���������С����ʵ�����ݼ�����Щ������̼��Ƶ�������������д��������̣�
�⣺��μӷ�Ӧ����������Ϊx��
CaCO3+ HCl===CaCl2+H2O+CO2��
36.5 28
x 1.1L
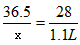
x=3g
�𣺲μӷ�Ӧ����������Ϊ3g��
��1��С���ļ�������г��ֶദ���������г����е���������
�� ���� ��
��2���������С����ʵ�����ݼ�����Щ������̼��Ƶ�������������д��������̣�
��1������ʽû����ƽ��
��CO2����Է���������������Ӧ����44��
������CO2�����1.1L���м��㣻
�������3g�������ѡ��������ɣ�
��2����õ�����̼��Ƶ�����Ϊx��
CO2��������1.1L��2g/L=2.2g
CaCO3+ 2HCl===CaCl2+H2O+CO2��
100 44
x 2.2g

x = 5g
������̼��Ƶ�����������5g �� 7.5g �� 100% = 66.7%
�𣺵�����̼��Ƶ���������Ϊ66.7%��
��CO2����Է���������������Ӧ����44��
������CO2�����1.1L���м��㣻
�������3g�������ѡ��������ɣ�
��2����õ�����̼��Ƶ�����Ϊx��
CO2��������1.1L��2g/L=2.2g
CaCO3+ 2HCl===CaCl2+H2O+CO2��
100 44
x 2.2g

x = 5g
������̼��Ƶ�����������5g �� 7.5g �� 100% = 66.7%
�𣺵�����̼��Ƶ���������Ϊ66.7%��

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ