��Ŀ����
һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ģ�ú��������Ҫ��Ӧ�У�
��1��2C+O2=2CO
��2��C+H2O=CO+H2
��3��CO+H2O=CO2+H2
A��������Ӧ���ڻ��Ϸ�Ӧ���� _________ ������ţ���
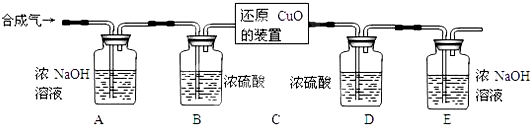
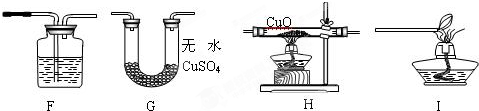
B���ϳ�����ͨ����Ȼ���������õ�����CH4+H2O=CO+3H2�ϳ������ƶ����ѣ������ѱ���Ϊ21���͵�����ȼ�ϣ��ϳ�����������ұ������������ұ�����IJ�����������ʾ����ͼ��
��1��2C+O2=2CO
��2��C+H2O=CO+H2
��3��CO+H2O=CO2+H2
A��������Ӧ���ڻ��Ϸ�Ӧ���� _________ ������ţ���
B���ϳ�����ͨ����Ȼ���������õ�����CH4+H2O=CO+3H2�ϳ������ƶ����ѣ������ѱ���Ϊ21���͵�����ȼ�ϣ��ϳ�����������ұ������������ұ�����IJ�����������ʾ����ͼ��
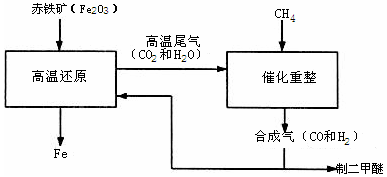
��1�������ѣ�CH3OCH3�����ɺϳ�����CO��H2����һ�����������Ƶģ��úϳ����ƶ�����ʱ����������һ�ֿɲ������ѭ���ġ�����ΪҺ̬�������д���÷�Ӧ�Ļ�ѧ����ʽ�� _________ ��
��2���ϳ�����ұ��������������������������� _________ ��
��2���ϳ�����ұ��������������������������� _________ ��
A����1��
B����1��2CO+4H2 CH3OCH3+H2O
CH3OCH3+H2O
��2������������ʯ�л�ԭ��������ԭ����
B����1��2CO+4H2
 CH3OCH3+H2O
CH3OCH3+H2O��2������������ʯ�л�ԭ��������ԭ����

��ϰ��ϵ�д�
�����Ŀ
 һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ģ�ú��������Ҫ��Ӧ�У�
һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ģ�ú��������Ҫ��Ӧ�У�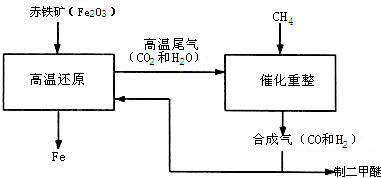
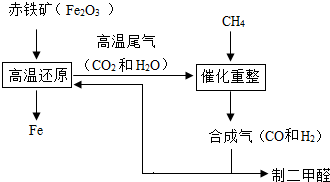
 ��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣�
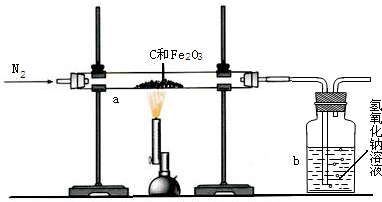
��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣� ��1�����ø�¯β���е�ˮ���������������õ��ϳ�������ѧ����ʽΪ
��1�����ø�¯β���е�ˮ���������������õ��ϳ�������ѧ����ʽΪ