ΧβΡΩΡΎ»ί
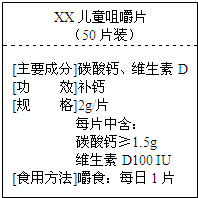 »γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬ΧβΘ°
»γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬ΧβΘ°Θ®1Θ©ΟΩΤ§ΗΤΤ§÷–÷Ν…ΌΚ§ΗΤ‘ΣΥΊΒΡ÷ ΝΩΈΣ
”―«ιΧα–―ΘΚΔΌΗΤΤ§÷–ΒΡΗΤ‘ΣΥΊ÷Μ¥φ‘Ύ”ΎΧΦΥαΗΤΘ®CaCO3Θ©÷–Θ§
ΔΎ÷Μ“Σ–¥≥ωΦΤΥψ ΫΘ§≤Μ“ΣΥψ≥ωΫαΙϊΘ°
Θ®2Θ©–ΓΜ®Ά§―ßΈΣ≤βΕ®Τδ÷–ΧΦΥαΗΤΒΡΚ§ΝΩ±ξΉΔ «Ζώ τ ΒΘ§
Υΐ»Γ≥ω10Τ§ΗΤΤ§Θ§―–ΥιΚσΖ≈»κ–Γ…’±≠÷–Θ§‘ΌΦ”»κΉψΝΩΒΡœΓ―ΈΥαΘ®HClΘ©Θ§≤βΒΟΖ¥”ΠΚσ…ζ≥…Εΰ―θΜ·ΧΦΒΡ÷ ΝΩΈΣ4.4ΩΥΘ°
«κΆ®ΙΐΦΤΥψ≈–ΕœΗΟΗΤΤ§÷–ΧΦΥαΗΤΘ®CaCO3Θ©ΒΡΚ§ΝΩ±ξΉΔ «Ζώ τ ΒΘ°
Θ®ΧΦΥαΗΤ”κœΓ―ΈΥαΖ¥”ΠΘΚCaCO3+2HCl=CaCl2+H2O+CO2ΓϋΘ©
Ζ÷ΈωΘΚΘ®1Θ©ΟΩΤ§ΗΤΤ§÷–÷Ν…ΌΚ§ΗΤ‘ΣΥΊΒΡ÷ ΝΩ=ΟΩΤ§ΗΤΤ§÷–Κ§ΧΦΥαΗΤΒΡ÷ ΝΩΓΝΧΦΥαΗΤ÷–ΗΤ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΘΜ
Θ®2Θ©ΗυΨίΜ·―ßΖΫ≥Χ ΫΚΆ…ζ≥…ΒΡΕΰ―θΜ·ΧΦΒΡ÷ ΝΩΘ§Φ¥Ω…ΦΤΥψ≥ω10Τ§ΗΤΤ§÷–Κ§ΧΦΥαΗΤΒΡ÷ ΝΩΘ§»ΜΚσΚΆ±ξ«©Υυ ΨΒΡ ΐΨίΫχ––±»ΫœΘ§ΨΆΩ…÷ΣΒάΗΟΗΤΤ§÷–ΧΦΥαΗΤΘ®CaCO3Θ©ΒΡΚ§ΝΩ±ξΉΔ «Ζώ τ ΒΘ°
Θ®2Θ©ΗυΨίΜ·―ßΖΫ≥Χ ΫΚΆ…ζ≥…ΒΡΕΰ―θΜ·ΧΦΒΡ÷ ΝΩΘ§Φ¥Ω…ΦΤΥψ≥ω10Τ§ΗΤΤ§÷–Κ§ΧΦΥαΗΤΒΡ÷ ΝΩΘ§»ΜΚσΚΆ±ξ«©Υυ ΨΒΡ ΐΨίΫχ––±»ΫœΘ§ΨΆΩ…÷ΣΒάΗΟΗΤΤ§÷–ΧΦΥαΗΤΘ®CaCO3Θ©ΒΡΚ§ΝΩ±ξΉΔ «Ζώ τ ΒΘ°
Ϋβ¥πΘΚΫβΘΚΘ®1Θ©ΧΦΥαΗΤ÷–ΗΤ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐ=1.5gΓΝ
ΓΝ100%ΘΜ
Ι ¥πΑΗΈΣΘΚ1.5gΓΝ
ΓΝ100%ΘΜ
Θ®2Θ©…η10Τ§ΗΤΤ§÷–Κ§ΧΦΥαΗΤΒΡ÷ ΝΩΈΣxΘ§
CaCO3+2HClΓζCaCl2+H2O+CO2Γϋ
100 44
x 4.4g
Γύ100ΘΚ44=xΘΚ4.4g
Ϋβ÷°ΒΟΘΚx=10gΘ§
ΗυΨί±ξ«©Υυ ΨΘ§ΧΦΥαΗΤΓί1.5gΘ§Ρ«Ο¥10Τ§ΗΤΤ§÷–Κ§ΧΦΥαΗΤΒΡ÷ ΝΩ”ΠΈΣ1.5gΓΝ10=15gΘΨ10gΘ°
Ι ΗΟΗΤΤ§÷–ΧΦΥαΗΤΘ®CaCO3Θ©ΒΡΚ§ΝΩ±ξΉΔ≤Μ τ ΒΘ°
| 40 |
| 100 |
Ι ¥πΑΗΈΣΘΚ1.5gΓΝ
| 40 |
| 100 |
Θ®2Θ©…η10Τ§ΗΤΤ§÷–Κ§ΧΦΥαΗΤΒΡ÷ ΝΩΈΣxΘ§
CaCO3+2HClΓζCaCl2+H2O+CO2Γϋ
100 44
x 4.4g
Γύ100ΘΚ44=xΘΚ4.4g
Ϋβ÷°ΒΟΘΚx=10gΘ§
ΗυΨί±ξ«©Υυ ΨΘ§ΧΦΥαΗΤΓί1.5gΘ§Ρ«Ο¥10Τ§ΗΤΤ§÷–Κ§ΧΦΥαΗΤΒΡ÷ ΝΩ”ΠΈΣ1.5gΓΝ10=15gΘΨ10gΘ°
Ι ΗΟΗΤΤ§÷–ΧΦΥαΗΤΘ®CaCO3Θ©ΒΡΚ§ΝΩ±ξΉΔ≤Μ τ ΒΘ°
ΒψΤάΘΚ±ΨΧβ÷ς“ΣΩΦ≤ι―ß…ζ‘Υ”Ο‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΙΪ ΫΚΆΜ·―ßΖΫ≥Χ ΫΫχ––ΦΤΥψΒΡΡήΝΠΘ°

ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
 »γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬Ν–ΗςΧβΘ°
»γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬Ν–ΗςΧβΘ°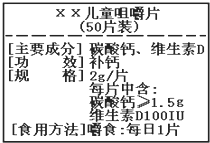 »γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬Ν–ΗςΧβΘ°÷ς“Σ≥…Ζ÷ΧΦΥαΗΤΒΡΜ·―ß Ϋ «CaCO3
»γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬Ν–ΗςΧβΘ°÷ς“Σ≥…Ζ÷ΧΦΥαΗΤΒΡΜ·―ß Ϋ «CaCO3 Μ·―ßΨΆ‘ΎΈ“Ο«…μ±ΏΘ§Υϋ”κΈ“Ο«ΒΡ…ζΜνœΔœΔœύΙΊΘ°
Μ·―ßΨΆ‘ΎΈ“Ο«…μ±ΏΘ§Υϋ”κΈ“Ο«ΒΡ…ζΜνœΔœΔœύΙΊΘ° »γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΘΚœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©
»γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΘΚœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ© »γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬Ν–ΗςΧβΘ°
»γΆΦΈΣΓΑßγßγΓ±ΗΤΤ§…ΧΤΖ±ξ«©ΆΦΘ§«κΗυΨί±ξ«©ΒΡ”–ΙΊ–≈œΔΆξ≥…œ¬Ν–ΗςΧβΘ°