��Ŀ����
ij��ѧ��ȤС����ͨ��ʵ��ⶨij��ʯ��ʯ��̼��Ƶ�����������ȡ��ʯ��ʯ��Ʒ8.0g����100mLϡ������Ĵμ��룬�����������±�����֪��ʯ��ʯ�е����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ����
��1���ϱ��У�m=______��n=______��
��2����ʯ��ʯ��̼��Ƶ���������Ϊ______��
��3����Ҫ��ȡ6.6g������̼��������Ҫ��ʯ��ʯ���ٿˣ�����д����ϸ�ļ�����̣��������һλС����
| ��� | ����ϡ����������mL�� | ��ַ�Ӧ��ʣ������������g�� |
| 1 | 25 | 5.5 |
| 2 | 25 | m |
| 3 | 25 | 1.2 |
| 4 | 25 | n |
��2����ʯ��ʯ��̼��Ƶ���������Ϊ______��
��3����Ҫ��ȡ6.6g������̼��������Ҫ��ʯ��ʯ���ٿˣ�����д����ϸ�ļ�����̣��������һλС����
��1����һ�μ���25mL�����ᣬʣ����������Ϊ5.5g��������������8g-5.5g=2.5g��˵��25mL������ȫ��Ӧ�ɷ�Ӧ2.5g̼��ƣ��ʵڶ���ʣ����������ӦΪ5.5g-2.5g=3.0g�������μ���25mL���ᣬʣ����������ֻ����3g-1.2g=1.8g��˵��̼���ȫ���μӷ�Ӧ����˵��Ĵμ��������ʣ������������䣮
�ʴ�Ϊ��3.0 1.2
��2����ʯ��ʯ��̼��Ƶ���������Ϊ��
��100%=85%
�ʴ�Ϊ��85%
��3����������Ҫ̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 6.6g
=
x=15.0g
��Ҫʯ��ʯ������Ϊ��15.0g��85%�T17.6g
��������Ҫ��ʯ��ʯ17.6g��
�ʴ�Ϊ��3.0 1.2
��2����ʯ��ʯ��̼��Ƶ���������Ϊ��
| 8.0g-1.2g |
| 8.0g |
�ʴ�Ϊ��85%
��3����������Ҫ̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 6.6g
| 100 |
| 44 |
| x |
| 6.6g |
x=15.0g
��Ҫʯ��ʯ������Ϊ��15.0g��85%�T17.6g
��������Ҫ��ʯ��ʯ17.6g��

��ϰ��ϵ�д�
�����Ŀ
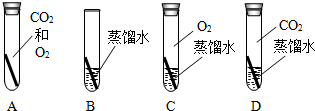
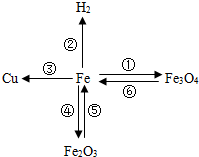 ����֪ʶ������һ����Ҫ��ѧϰ��������ͼ���й��������ʺͻ�õ�����ͼ��
����֪ʶ������һ����Ҫ��ѧϰ��������ͼ���й��������ʺͻ�õ�����ͼ�� ����ΪͭƬ����ֻ�����ʵ��
����ΪͭƬ����ֻ�����ʵ��
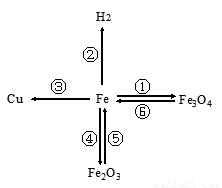
 ����ΪͭƬ����ֻ�����ʵ�� �� ���ɴﵽ̽��Ŀ�ģ�ѡ����ĸ����
����ΪͭƬ����ֻ�����ʵ�� �� ���ɴﵽ̽��Ŀ�ģ�ѡ����ĸ����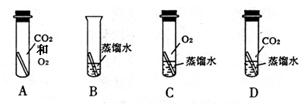
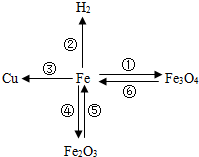 ����֪ʶ������һ����Ҫ��ѧϰ��������ͼ���й��������ʺͻ�õ�����ͼ��
����֪ʶ������һ����Ҫ��ѧϰ��������ͼ���й��������ʺͻ�õ�����ͼ�� ����ΪͭƬ����ֻ�����ʵ��______��______���ɴﵽ̽��Ŀ�ģ�ѡ����ĸ����
����ΪͭƬ����ֻ�����ʵ��______��______���ɴﵽ̽��Ŀ�ģ�ѡ����ĸ����