��Ŀ����
���ڳ�ʪ�Ŀ������������⣮
��1����ֹ������Ĵ�ʩ�� _________ ��дһ�㼴�ɣ�����ԭ���� _________ ��
��2���������Ҫ�ɷ��� _________ ����һ�������������ϡ�����г��⣬�۲쵽����������Һ��Ϊ _________ ɫ��ͬʱ����������ð����д��������Ӧ�Ļ�ѧ����ʽ�� _________ �� _________ ��
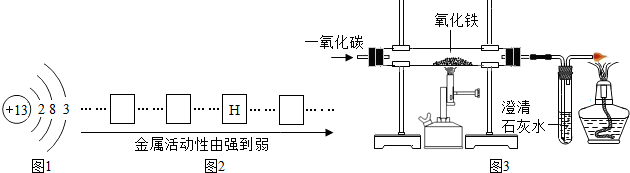
��3���������������������Ʒ�DZ���������Դ��һ����Ч;������ͼ��ʾ�ķ�������Fe2O3������Լ��80%������20%Ϊ�������ң����պ��ڹ�ҵ�ϳ���һ����̼���仹ԭ����Ӧ�Ļ�ѧ����ʽ�� _________ ��
��1����ֹ������Ĵ�ʩ�� _________ ��дһ�㼴�ɣ�����ԭ���� _________ ��
��2���������Ҫ�ɷ��� _________ ����һ�������������ϡ�����г��⣬�۲쵽����������Һ��Ϊ _________ ɫ��ͬʱ����������ð����д��������Ӧ�Ļ�ѧ����ʽ�� _________ �� _________ ��
��3���������������������Ʒ�DZ���������Դ��һ����Ч;������ͼ��ʾ�ķ�������Fe2O3������Լ��80%������20%Ϊ�������ң����պ��ڹ�ҵ�ϳ���һ����̼���仹ԭ����Ӧ�Ļ�ѧ����ʽ�� _________ ��

��1��ˢ�������������ˮ
��2�����������ƣ�
Fe2O3+6HCl==2FeCl3+3H2O��Fe+2HCl==FeCl2+H2��
��3��3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2
��2�����������ƣ�
Fe2O3+6HCl==2FeCl3+3H2O��Fe+2HCl==FeCl2+H2��
��3��3CO+Fe2O3
 2Fe+3CO2
2Fe+3CO2
��ϰ��ϵ�д�
�����Ŀ