��Ŀ����
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g�����ձ��У���80g9.8%��ϡ������Ĵμ�����ձ��У���ַ�Ӧ���ʣ������������ݼ�¼���£�| ���� | 1 | 2 | 3 | 4 |
| ����ϡ���������/g | 20.0 | 20.0 | 20.0 | 20.0 |
| ʣ����������/g | 8.7 | 7.4 | 6.1 | 5.8 |
��1����Ʒ��ͭ������������
��2����Ӧ�����ĵ�������Һ������
��3�������μ��������ַ�Ӧ��������Һ�����ʵ�����������
���������ݽ����Ļ�Կ�֪����ͭ��ͭ������ϡ���ᷢ����Ӧ����п����ϡ����ų���������п��ϡ���ᷢ����Ӧ��ʣ������������ϼ��٣�
��1���ɵ�1��ʵ�����ݣ�����20gϡ���������������1.3g��˵��20gϡ������ȫ��Ӧ������1.3gп��
��2���ԱȺ����ʵ�����ݿɷ��֣���4�μ���20gϡ����ʱ����������ֻ����0.3g����1.3g������˵����ʱ��ͭ�е�п����ȫ��Ӧ������ʣ�����Ϊ����ϡ���ᷴӦ��ͭ�����ݷ�Ӧ�Ļ�ѧ����ʽ�����ɷ�Ӧ����п����������μӷ�Ӧ��ϡ�����������
��3��ǰ���η�Ӧ��ϡ���ᶼ��ȫ��Ӧ�����ԣ������μ��������ַ�Ӧ��������ҺΪ����п��Һ�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������������������п��������������Һ�ıȼ���Һ�����ʵ�����������
��1���ɵ�1��ʵ�����ݣ�����20gϡ���������������1.3g��˵��20gϡ������ȫ��Ӧ������1.3gп��
��2���ԱȺ����ʵ�����ݿɷ��֣���4�μ���20gϡ����ʱ����������ֻ����0.3g����1.3g������˵����ʱ��ͭ�е�п����ȫ��Ӧ������ʣ�����Ϊ����ϡ���ᷴӦ��ͭ�����ݷ�Ӧ�Ļ�ѧ����ʽ�����ɷ�Ӧ����п����������μӷ�Ӧ��ϡ�����������
��3��ǰ���η�Ӧ��ϡ���ᶼ��ȫ��Ӧ�����ԣ������μ��������ַ�Ӧ��������ҺΪ����п��Һ�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������������������п��������������Һ�ıȼ���Һ�����ʵ�����������
����⣺��1����ͭ��Ʒ��ͭ����������=
��100%=58.0%��
�ʴ�Ϊ��58.0%��
��2���μӷ�Ӧп������=10g-5.8g=4.2g
�蹲���ĵ�������Һ����Ϊx
Zn+H2SO4�TZnSO4+H2��
65 98
4.2g 9.8%x
=
x=64.6g
�𣺹�����������Һ��������64.6g��
��3���μӷ�Ӧп������=10g-6.1g=3.9g
��ǰ���η�Ӧ��������п������Ϊy���ų���������Ϊz
Zn+H2SO4�TZnSO4+H2��
65 161 2
3.9g y z
=
y=9.66g
=
z=0.12g
������Һ�����ʵ���������=
��100%��15.1%
�ʴ�Ϊ��15.1%��
| 5.8g |
| 10g |
�ʴ�Ϊ��58.0%��
��2���μӷ�Ӧп������=10g-5.8g=4.2g
�蹲���ĵ�������Һ����Ϊx
Zn+H2SO4�TZnSO4+H2��
65 98
4.2g 9.8%x
| 65 |
| 4.2g |
| 98 |
| 98%X |
�𣺹�����������Һ��������64.6g��
��3���μӷ�Ӧп������=10g-6.1g=3.9g
��ǰ���η�Ӧ��������п������Ϊy���ų���������Ϊz
Zn+H2SO4�TZnSO4+H2��
65 161 2
3.9g y z
| 65 |
| 3.9g |
| 161 |
| y |
| 65 |
| 3.9g |
| 2 |
| z |
������Һ�����ʵ���������=
| 9.66g |
| 3.9g+60g-0.12g |
�ʴ�Ϊ��15.1%��
���������������غ㶨�ɣ�������ʵ���������Һ����=�μӷ�Ӧп������+����ʵ������ϡ��������-�ų�������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
ij��ѧ��ȤС��Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ���п������������ȡ��6g�Ͻ���Ʒ����50gϡ�����5�μ�����Ʒ�У���ַ�Ӧ����ˡ�ϴ�ӡ�������أ��õ���ʵ���������£�
��1��mֵΪ ��
��2����Ͻ���п������������
��3����ϡ���������ʵ�����������
| ϡ�������� | ʣ��������� |
| ��һ�μ���10g | 4.7g |
| �ڶ��μ���10g | mg |
| �������10g | 2.1g |
| ���Ĵμ���10g | 1.2g |
| ������10g | 1.2g |
��2����Ͻ���п������������
��3����ϡ���������ʵ�����������
 Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺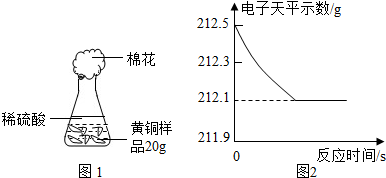
 Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ�ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ����������������������ϵ��ͼ��ʾ������ͼʾ�ش����⣺
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ�ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ����������������������ϵ��ͼ��ʾ������ͼʾ�ش����⣺