��Ŀ����
 ʳƷ��˾������ѩ�����İ�װ���ڷ���һС���������������д��˵��������ϸ�Ķ������ش��������⣮
ʳƷ��˾������ѩ�����İ�װ���ڷ���һС���������������д��˵��������ϸ�Ķ������ش��������⣮������������ˮ�ֵĻ�ѧ����ʽΪ
CaO+H2O=Ca��OH��2
CaO+H2O=Ca��OH��2
�����ָ����ԭ����Ũ����ĸ���ԭ���кβ�ͬ��Ũ����������ˮ�֣�����ʯ���Ǻ�ˮ��Ӧ
Ũ����������ˮ�֣�����ʯ���Ǻ�ˮ��Ӧ
��������ʳ�á�����Ϊ�������ˮ�����
��ʴ�ԣ����ԣ�
��ʴ�ԣ����ԣ�
�ԣ����������ݸ��������Ҫ�ɷ�����ʯ�ң�CaO��������ˮ�ֺ�������������Ca��OH��2���ݴ�д����ѧ����ʽ���������ˮ����������ƣ�����������ǿ���ԣ���Ƥ����֯�����������и�ʴ���ã�
����⣺���������Ҫ�ɷ�����ʯ�ң�CaO��������ˮ�ֺ�������������Ca��OH��2����ѧ����ʽΪCaO+H2O=Ca��OH��2��Ũ�������������Ũ����������ˮ�֣�������ʯ���Ǻ�ˮ��Ӧ����������������ˮ����������ƣ�����������ǿ���ԣ���Ƥ����֯�����������и�ʴ���ã�
�ʴ�Ϊ��CaO+H2O=Ca��OH��2��Ũ����������ˮ�֣�����ʯ���Ǻ�ˮ��Ӧ����ʴ�ԣ����ԣ���
�ʴ�Ϊ��CaO+H2O=Ca��OH��2��Ũ����������ˮ�֣�����ʯ���Ǻ�ˮ��Ӧ����ʴ�ԣ����ԣ���
������������Ҫ����ѧ���������ƺ��������Ƶ����ʵ���ʶ��

��ϰ��ϵ�д�
�����Ŀ
 21��ʳƷ��˾ͨ���ڡ�����ѩ�����İ�װ���ڷ���һС�������������IJ���������ͼ��
21��ʳƷ��˾ͨ���ڡ�����ѩ�����İ�װ���ڷ���һС�������������IJ���������ͼ�� 29��ʳƷ��˾ͨ���ڡ�XXѩ�����İ�װ���ڷ���һС���������������IJ������ּ�ͼ������ϸ�Ķ����ش����⣺
29��ʳƷ��˾ͨ���ڡ�XXѩ�����İ�װ���ڷ���һС���������������IJ������ּ�ͼ������ϸ�Ķ����ش����⣺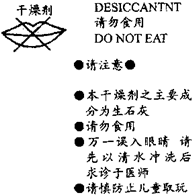 ����ʳƷ��˾�ڡ�����ѩ�����İ�װ���ڶ�����һС�������������IJ������ּ���ͼ���뿴ͼ�ش��������⣺
����ʳƷ��˾�ڡ�����ѩ�����İ�װ���ڶ�����һС�������������IJ������ּ���ͼ���뿴ͼ�ش��������⣺