��Ŀ����
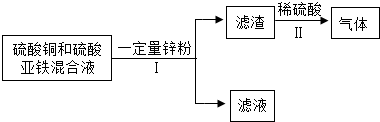
�����ַ�Һ����Ҫ�ɷֱַ���HCl��NaOH��CuCl2��Na2CO3���ֽ���Һ������Ϊ���ţ���������ͼ��ʾ�Ĵ���������Һ��Ϊ��ɫ��Һ����ش�
�������⣺
��1�������ڵ�������______���ù������õ�����������Ϊ______��
��2����Һ�����Ҫ�ɷֵĻ�ѧʽΪ______���������ѧʽΪ______��
��3����Һ�����Һ��Ӧ�Ļ�ѧ����ʽΪ______��
��4������Һ�����ɫ������ҺA��B���ʱ��Ӧ�Ļ�ѧ����ʽΪ��______��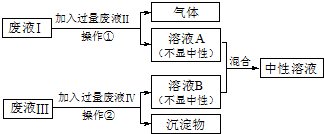
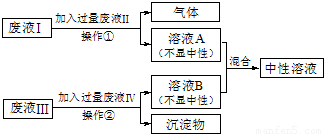
�������⣺
��1�������ڵ�������______���ù������õ�����������Ϊ______��
��2����Һ�����Ҫ�ɷֵĻ�ѧʽΪ______���������ѧʽΪ______��
��3����Һ�����Һ��Ӧ�Ļ�ѧ����ʽΪ______��
��4������Һ�����ɫ������ҺA��B���ʱ��Ӧ�Ļ�ѧ����ʽΪ��______��

��Һ��Ϊ��ɫ��Һ����˷�Һ��Ϊ�Ȼ�ͭ��Һ�������Һ������������Ͳ������Ե���ҺB����˷�Һ��Ϊ����������Һ��������Ϊ������ͭ����ҺB�к����Ȼ��ƺ��������ƣ���Һ�������Һ��Ӧ�������壬ͬʱ�õ���ҺA�������ԣ���˷�Һ��Ϊ���ᣬ��Һ��Ϊ̼���ƣ���ҺA�к����Ȼ��ƺ����ᣬ��ҺA�е��������ҺB�е��������Ʒ�Ӧ�������Ե��Ȼ��ƣ�
��1���������ǽ������������ҺB���룬��˿��� ���ˣ��ù������õ�����������Ϊ ����̨��
��2��������������֪��Һ�����Ҫ�ɷ�Ϊ���ᣬ��ѧʽΪ HCl��������Ϊ�����������Ȼ�ͭ��Ӧ������������ͭ����ѧʽΪ Cu��OH��2��
��3��������������Һ��Ϊ̼���ƣ���Һ��Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ 2HCl+Na2CO3�T2NaCl+H2O+CO2����
��4��������������֪��ҺA�к������ᣬ��ҺB�к����������ƣ����ʱ��Ӧ�Ļ�ѧ����ʽΪ��HCl+NaOH�TNaCl+H2O
�ʴ�Ϊ����1������ ����̨ ��2��HCl Cu��OH��2
��3��2HCl+Na2CO3�T2NaCl+H2O+CO2��
��4��HCl+NaOH�TNaCl+H2O
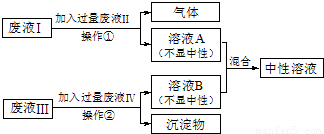
��1���������ǽ������������ҺB���룬��˿��� ���ˣ��ù������õ�����������Ϊ ����̨��
��2��������������֪��Һ�����Ҫ�ɷ�Ϊ���ᣬ��ѧʽΪ HCl��������Ϊ�����������Ȼ�ͭ��Ӧ������������ͭ����ѧʽΪ Cu��OH��2��
��3��������������Һ��Ϊ̼���ƣ���Һ��Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ 2HCl+Na2CO3�T2NaCl+H2O+CO2����
��4��������������֪��ҺA�к������ᣬ��ҺB�к����������ƣ����ʱ��Ӧ�Ļ�ѧ����ʽΪ��HCl+NaOH�TNaCl+H2O
�ʴ�Ϊ����1������ ����̨ ��2��HCl Cu��OH��2
��3��2HCl+Na2CO3�T2NaCl+H2O+CO2��
��4��HCl+NaOH�TNaCl+H2O

��ϰ��ϵ�д�
�����Ŀ
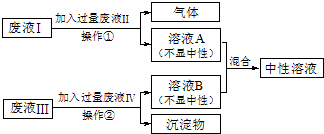 �����ַ�Һ����Ҫ�ɷֱַ���HCl��NaOH��CuCl2��Na2CO3���ֽ���Һ������Ϊ���ţ���������ͼ��ʾ�Ĵ���������Һ��Ϊ��ɫ��Һ����ش�
�����ַ�Һ����Ҫ�ɷֱַ���HCl��NaOH��CuCl2��Na2CO3���ֽ���Һ������Ϊ���ţ���������ͼ��ʾ�Ĵ���������Һ��Ϊ��ɫ��Һ����ش�