��Ŀ����
��5�֣�A��B��C��D��EΪ���л�ѧ�������ʣ�����֮������ͼ1��ʾ��ת����ϵ����������ʾ���ɣ�������E�����ڼ�������B���ش��������⣺

��1��E���ʵĻ�ѧʽΪ ��
��2��A���������������й㷺��Ӧ�ã��Ծ�һ��˵�� ��
��3��D��A�Ļ�ѧ����ʽΪ ��
��4��C��D�Ļ�ѧ����ʽΪ ��
��5����ͼ2��ʾװ�ý���ʵ�飬��ʵ��D��E��ת�����۲쵽U��a��Һ�����Խ��ͣ�������һ�����ԭ���� ��
���𰸡�
��5�֣�ÿ��1�֣����������𰸵÷�
��1��Ca��OH��2 �� ��2�����Ȳ���
��3��2H2O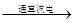 2H2��+O2��
2H2��+O2��
��4��CaCO3+2HCl = CaCl 2+CO2��+H2O
��5��CaO+H2O= Ca��OH��2�ų�������ʹ����ƿ��������Ӽ��϶��Ӷ��������U����Һ������ƫת��
����������

��ϰ��ϵ�д�
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
�����Ŀ
 A��B��C��D��EΪ���л�ѧ���������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ���л�ѧ���������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش� 16��A��B��C��D��EΪ���л�ѧ���������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
16��A��B��C��D��EΪ���л�ѧ���������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
