��Ŀ����
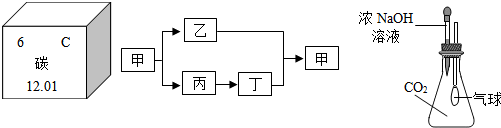
�ס��ҡ��������dz��л�ѧ�ij������ʣ���������ͼ��ת����ϵ��

(1)��������������壬�ǹ��ϵ������ȼ�ϣ�д�����ϴ�ת����ϵ��
һ����ѧ����ʽ��_________________________��
(2)�����Ǽ�����Ѫ�쵰��ϵ��ж����壬����һ������������ҹ����ڴ���ս��ʱ�ھͿ�ʼ������ʹ�õ�һ�ֽ������ʣ�д�����ϴ�ת����ϵ��һ����ѧ����ʽ��______________________________��
(3)�������ɵ��۷��Ͷ��õ�һ��Һ̬��������Դ��������ʹ����ʯ��ˮ����ǵ����壬д�����ϴ�ת����ϵ��һ����ѧ����ʽ��___________________________��
(1)Zn+2HCl��ZnCl2+H2����(2�֣�������ȷ�𰸲��ո���)
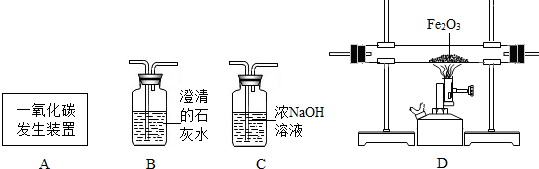
(2)3CO+Fe2O3���� 2Fe+3CO2�� (2��)
(3)C2H5OH+3O2�� ![]() �� 2CO2+3H2O(2��)
�� 2CO2+3H2O(2��)

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �ס��ҡ��������dz��л�ѧ�ij������ʣ���������ͼ��ת����ϵ��
�ס��ҡ��������dz��л�ѧ�ij������ʣ���������ͼ��ת����ϵ��