��Ŀ����
�����������̽���������龰��ij��ѧʵ��С��ͬѧ������Ͷ������ͭ��Һ�У��������ɺ�ɫ�������ʵ�ͬʱ���н϶�����ݷų�����һ������ͬѧ�ǵ�̽�����������ɵ���ʲô�����أ�
������룺���������Ԫ�صĽǶȷ������ų������������SO2��O2��H2��
�������ϣ�SO2������ˮ��������NaOH��Һ��Ӧ������Na2SO3��
������ƣ������������룬ʵ��С��ͬѧ�ֱ���������·�����
��1����ͬѧ��Ϊ��O2������鷽����
��2����ͬѧ��Ϊ��SO2����ֻ�轫�ų�������ͨ��ʢ��NaOH��Һ��ϴ��ƿ�У�����ͨ��ǰ��ϴ��ƿ��������д��SO2��NaOH��Ӧ�Ļ�ѧ����ʽ��
��3��ʵ��С��ͬѧ����������������ʵ�鷽�����ų�����֤��������壮
����ų�����O2��H2�Ļ�������Ϊ�����еİ�ȫ������
��ͬѧ��Ϊ��Ϊȷ����ȫ��ʵ��ǰӦ���ռ�һ�Թ����壬��Ĵָ
ʵ��̽����
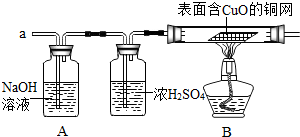
����ͬѧȷ�ϰ�ȫ��С��ͬѧ���ȶ�װ��A���г������ٽ��ռ����������aͨ�룬��һ�����ȼB���ƾ��ƣ�һ��ʱ����ֱ��溬CuO��ͭ���ɺ�ɫ��Ϊ�����ĺ�ɫ��ֹͣͨ�����ٴγ���װ��A�������������䣮
���ۣ�
��1������������ͭ��Һ��Ӧʱ��������������
��2��д��H2��ԭCuO�Ļ�ѧ����ʽ��
˼ά��չ��
��1��������ʵ������Ƴ�������ͭ��Һ��
��2����ͨ������������ж�SO2��NaOH��Һ�����˷�Ӧ�أ���һͬѧ�������ͼ��ʾ��װ�ý���ʵ�飬�����Թ���Һ���������͵ó�SO2��NaOH��Һ������Ӧ�Ľ��ۣ�����ͬѧ��Ϊ��һ�������Ͻ���������
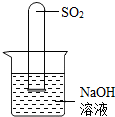
��3��������ͼ�Թ���SO2��Ӧ��ȫ����NaOH��ʣ�࣬��ʱ��Һ�д��ڵ���������Щ��
������[�������]��1����֤�Ƿ�Ϊ���������������ķ�������2������ͨ��ǰ��ϴ��ƿ����������֤�Ƿ��ж����������ɣ����ݷ�Ӧ����������ȷ��д��ѧ����ʽ����3����ȼ�����岻���ᱬը��
[ʵ��̽��]��1����Ϊ�����ֱ��溬CuO��ͭ���ɺ�ɫ��Ϊ�����ĺ�ɫ��ֹͣͨ�����ٴγ���װ��A�������������䡱�����Բ���������Ϊ��������2����д������ԭ����ͭ�Ļ�ѧ����ʽ��
[˼ά��չ]��1����Ϊ���������ɣ�����Ҫ������������Ӵ��ڣ���2����������������������Һ��Ӧ������ˮ��Ӧ����3��������Һ�е����ӻش�
[ʵ��̽��]��1����Ϊ�����ֱ��溬CuO��ͭ���ɺ�ɫ��Ϊ�����ĺ�ɫ��ֹͣͨ�����ٴγ���װ��A�������������䡱�����Բ���������Ϊ��������2����д������ԭ����ͭ�Ļ�ѧ����ʽ��
[˼ά��չ]��1����Ϊ���������ɣ�����Ҫ������������Ӵ��ڣ���2����������������������Һ��Ӧ������ˮ��Ӧ����3��������Һ�е����ӻش�
����⣺[�������]��1��ע������ļ��鷽������2������Ϊ��������ͨ������ͨ��ǰ��ϴ��ƿ��������
��3����ȼ�����岻���ᱬը��ʵ��ʱ��B��������ը�¹ʣ�
[ʵ��̽��]��1��ͨ��������֪������֪����������Ϊ��������2����д������ԭ����ͭ�Ļ�ѧ����ʽCuO+H2=Cu+H2O
[˼ά��չ]��1����Ϊ���������ɣ�����Ҫ������������Ӵ��ڣ���֪����ͭ��Һ�����ԣ�
��2��Ϊ�˸��Ͻ���Ӧ������������Һ����ˮ��
��3��ʣ����Һ���������ƺ��������ƵĻ������е����Ӿ��������ӡ����������ӡ�����������ӣ�ˮ���ӵ�
�ʴ�Ϊ��
������ƣ�
��1���ô����ǵ�ľ�����飬��ľ���Ƿ�ȼ
��2��SO2+2NaOH=Na2SO3+H2O
��3��ʵ��ʱ��B��������ը�¹ʣ���ס�Թ��������������𣬼���ı�����
ʵ��̽����
��1��H2
��2��H2+CuO
Cu+H2O
˼ά��չ��
��1����
��2��SO2������ˮ��Ҳ��ʹҺ������
��3��Na+��OH-��SO32-��H2O���˿���һ�����ӿ�0.5�֣��ٶ���ȫ�ۣ�
��3����ȼ�����岻���ᱬը��ʵ��ʱ��B��������ը�¹ʣ�
[ʵ��̽��]��1��ͨ��������֪������֪����������Ϊ��������2����д������ԭ����ͭ�Ļ�ѧ����ʽCuO+H2=Cu+H2O
[˼ά��չ]��1����Ϊ���������ɣ�����Ҫ������������Ӵ��ڣ���֪����ͭ��Һ�����ԣ�
��2��Ϊ�˸��Ͻ���Ӧ������������Һ����ˮ��
��3��ʣ����Һ���������ƺ��������ƵĻ������е����Ӿ��������ӡ����������ӡ�����������ӣ�ˮ���ӵ�
�ʴ�Ϊ��
������ƣ�
��1���ô����ǵ�ľ�����飬��ľ���Ƿ�ȼ
��2��SO2+2NaOH=Na2SO3+H2O
��3��ʵ��ʱ��B��������ը�¹ʣ���ס�Թ��������������𣬼���ı�����
ʵ��̽����
��1��H2
��2��H2+CuO
| ||
˼ά��չ��
��1����
��2��SO2������ˮ��Ҳ��ʹҺ������
��3��Na+��OH-��SO32-��H2O���˿���һ�����ӿ�0.5�֣��ٶ���ȫ�ۣ�
������������Ҫ���黯ѧʵ��ķ�����������ۣ��˽�������ʵ��֤�ķ�����������ը�Ĵ�ʩ�����������ļ��鷽����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ



