��Ŀ����
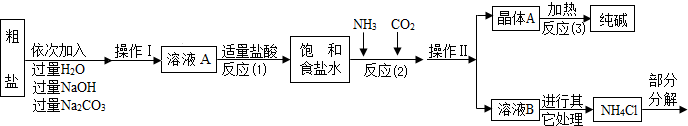
��ͼ��ʾ����ijͬѧ��Ƶ��ø��������ȡ���ռ�������װ��ͼ������ͼ�λش��й����⣺

��1��ָ��ͼ���е����Դ���
��___________________����_____________________��
��___________________����_____________________��
��2��ָ��ͼ�����б�ŵ����������ƣ�
��___________________����_____________________��
��___________________����_____________________��
�������������ʵ�飺
��3����������װ�������صķ�����___________________________��Ŀ����______________________________________��
��4�����Թܼ��ȵķ�����ȷ���ǣ�Ӧ��_____________________��Ȼ��_________________________��Ŀ����__________________________________________��
��5���ռ����������˵�ʱ����______________________����ԭ����_________________��
��6���ж�����ƿ�ռ���������������_________________________________________��
��7���ռ�������ϣ�ֹͣʵ��ʱ��Ӧ��________________________________����_____________________�������________________________________________��
��1���ٲ�Ӧ�þƾ���������� ���Թܿڲ�Ӧ������б �����в�Ӧ�����Թ��в� �ܵ��ܿ�δ����
��2���پƾ��� ������̨ ���Թ� ��ˮ��
��3�����Թ��� б����ҩ��ҩƷ�����Թܵײ���ֱ���Թ� ��ֹҩƷճ���Թܱ���
��4����Ԥ���Թ� �̶����Թܵײ���ҩƷ���� ��ֹ�Թֲܾ��¶ȹ���ը��
��5��������������ð�� ��ʼð�������嶼�ǿ���
��6��ˮ���У�����ƿ�ڱ�Ե���д�����ð��
��7��ȡ������ Ϩ��ƾ��� ˮ�������Թ������Թ�ը��
������������������������⣬���ͼʾ������
��1��ͼ�еĴ���ӷ�Ӧװ��ͼ���Կ������پƾ��������¶ȸߣ���������ȶ���Ӧ�þƾ���������ȣ���Ϊ��ֹ�Թܿ���ˮ�����ɲ������Թܵײ����Թܿ�Ӧ������б���Թܿڲ�Ӧ������б��������Ӧ��ס���Թܿڵ�����֮һ��������Ӧ�����Թ��в�����Ϊ��ֹ������ؿ��������ռ��������У��Թܿ�Ӧ�����ţ���ͼ��װ�õĵ��ܿ�δ������
��2����Ţ٢ڢۢܵ��������Ʒֱ�Ϊ���پƾ��� ������̨ ���Թ� ��ˮ�ۡ�
��3��Ϊ��ֹҩƷճ���Թܱ��ϣ����Թ��ڷ����ĩ״ҩƷ�ķ����ǣ��Ƚ��Թܺ�ţ�����ҩ��ҩƷ�����Թܵײ������ֱ���Թܡ�
��4��Ϊ��ֹ�Թֲܾ��¶ȹ���ը�ѣ�Ӧ��Ԥ���Թܣ��ٹ̶����Թܵײ���ҩƷ���ȡ�
��5����ʼʱð�����������Թ��ڵĿ�����Ҫ����������ð��ʱ������ʼ�ų�����ʱ�����ռ����塣
��6������ƿ�ռ�������ʱˮ���У�����ƿ�ڱ�Ե���д�����ð����
��7��Ϊ��ֹ���¶��½���ѹǿ��С��ˮ�������Թ������Թ�ը�ѣ�Ӧ��ȡ�����ܣ���Ϩ��ƾ��ơ�
���㣺��������ȡװ�ã��������������ƺ�ѡ�ã���ȡ�����IJ��������ע���
�����������ȡ������ʵ�鲽�衢װ��ʾ��ͼ���������������ơ�ע��������ǽ������Ĺؼ���

 ijͬѧҪ���Ȼ��ƹ�������������������Ϊ16%��ʳ����Һ��
ijͬѧҪ���Ȼ��ƹ�������������������Ϊ16%��ʳ����Һ��