��Ŀ����
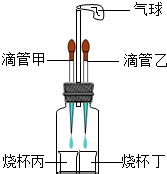 ��2012?�人ģ�⣩��ͼ��ʾװ�ã����������ã����ı�ιܺ�С�ձ��е����ʿ�����ɶ����ʵ�飮
��2012?�人ģ�⣩��ͼ��ʾװ�ã����������ã����ı�ιܺ�С�ձ��е����ʿ�����ɶ����ʵ�飮��1�����ιܼ�������ˮ���ձ�����ʢ��������ʯ�ң����ιܼ��е�ˮ�����ձ����У��ɹ۲쵽�������һ��ʱ���װ�ûָ�ԭ״��������ѧ��ѧ֪ʶ������������ԭ��
��ʯ�Һ�ˮ��Ӧ�ų��������ȣ�ʹװ����������������
��ʯ�Һ�ˮ��Ӧ�ų��������ȣ�ʹװ����������������
�����ձ�����ʢ������п�������ι����е�Һ ������ձ����У��ձ��������������ɣ��������Թ�����ι�������װҺ����ϡ����
ϡ����
���ձ����з�����Ӧ�Ļ�ѧ����ʽΪZn+2HCl=ZnCl2+H2��
Zn+2HCl=ZnCl2+H2��
����2�����ιܼ�������ϡ���ᣬ�ι���������ˮ���ձ�����ʢ������С�մ��ձ�����ʢ����ʯ����ҺȾ����ɫ�ĸ���ֽ������ʵ���������֤ʹʯ���ɫ��������̼������Ƕ�����̼����ȷ�IJ���˳���ǣ���
���ιܼ��е�ϡ��������ձ�����
���ιܼ��е�ϡ��������ձ�����
���۲��ձ����е�ֽ���Ƿ��ɫ
�ձ����е�ֽ���Ƿ��ɫ
��������ι����е�ˮ
����ι����е�ˮ
����������1��С�������������װ���е���ѹ�����µģ�������ʯ�ҵĻ�ѧ���ʡ������Ļ�ѧ���ʽ��з������
��2������̼�����ɶ�����̼��ˮ��Ӧ���ɵģ��Ȱ�ʯ����ҺȾ����ɫ�ĸ���ֽ���������̼�Ӵ������Ƿ��б仯��Ȼ����ʹʯ����ҺȾ����ɫ�ĸ���ֽ����ˮ�Ӵ����ٹ۲���仯��
��2������̼�����ɶ�����̼��ˮ��Ӧ���ɵģ��Ȱ�ʯ����ҺȾ����ɫ�ĸ���ֽ���������̼�Ӵ������Ƿ��б仯��Ȼ����ʹʯ����ҺȾ����ɫ�ĸ���ֽ����ˮ�Ӵ����ٹ۲���仯��
����⣺��1����ʯ�Һ�ˮ��Ӧ�ų���������ʹװ���������������ͣ��ʽ��ιܼ��е�ˮ�����ձ����У��ɹ۲쵽�������
п�ǽ��������Ա���ǿ�������ᷴӦ����������ʹװ����ѹǿ�����ʹ�������Թ��𣬷�Ӧ�Ļ�ѧ����ʽΪ��Zn+2HCl=ZnCl2+H2����
��2����֤ʹʯ���ɫ��������̼������Ƕ�����̼��Ҫ��ͨ��ϡ������С�մ�Ӧ����������̼��������ɫ�ĸ���ֽ������ɫ���ٵμ�ˮ���ɫ��˵��������̼��ˮ��Ӧ����̼�ᣬ̼��ʹʯ����Һ��죮
�ʴ�Ϊ����1����ʯ�Һ�ˮ��Ӧ�ų��������ȣ�ʹװ���������������ͣ�ϡ���Zn+2HCl=ZnCl2+H2����
��2�����ιܼ��е�ϡ��������ձ����У��ձ����е�ֽ���Ƿ��ɫ������ι����е�ˮ��
п�ǽ��������Ա���ǿ�������ᷴӦ����������ʹװ����ѹǿ�����ʹ�������Թ��𣬷�Ӧ�Ļ�ѧ����ʽΪ��Zn+2HCl=ZnCl2+H2����
��2����֤ʹʯ���ɫ��������̼������Ƕ�����̼��Ҫ��ͨ��ϡ������С�մ�Ӧ����������̼��������ɫ�ĸ���ֽ������ɫ���ٵμ�ˮ���ɫ��˵��������̼��ˮ��Ӧ����̼�ᣬ̼��ʹʯ����Һ��죮
�ʴ�Ϊ����1����ʯ�Һ�ˮ��Ӧ�ų��������ȣ�ʹװ���������������ͣ�ϡ���Zn+2HCl=ZnCl2+H2����
��2�����ιܼ��е�ϡ��������ձ����У��ձ����е�ֽ���Ƿ��ɫ������ι����е�ˮ��
������������һ���Ѷȣ�������������ԭ�������������ˮ�ų������ȣ������ճ�����������ˮ������������������ƵĻ�ѧ���ʵ�����ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
 ��2012?�人ģ�⣩ij������Ȼ��Ȫˮ��ǩ�IJ���������ͼ��ʾ�� ����ϸ�Ķ����ش��������⣮
��2012?�人ģ�⣩ij������Ȼ��Ȫˮ��ǩ�IJ���������ͼ��ʾ�� ����ϸ�Ķ����ش��������⣮