��Ŀ����
��8�֣���������CO2��������̼�����չ��̼�����ѳ�Ϊһ�ֻ���ʱ�С���̼������һ���Ե��ܺĺ�Ч�ܵ�Ϊ��Ҫ�������Խ��ٵ����������ŷŻ�ýϴ�������¾��÷�չģʽ�����ʣ�
(1)�����������ж�����̼����������������Ҫԭ���� ����Ȼ�������Ķ�����̼����Ҫ;���� ��
(2)�������������ϡ���̼���á�������ǣ�����ţ� ��
�ٸ������̭���ܺġ�����Ⱦ��ҵ���ڴ�����չ�������磻�����ƺͿ�������Դ�����ͳ��Դ�����Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ硣
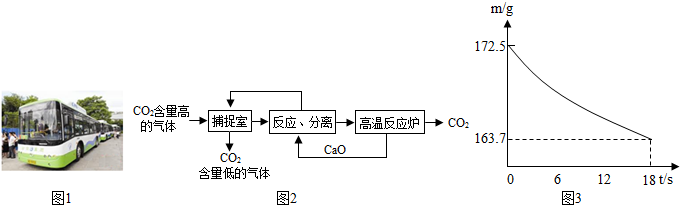
(3)Ŀǰ���ó��ٽ�CO2����״̬������̬��Һ̬֮�䣩�����������������ѳ�Ϊһ�����ƣ���һ�����Ի����Ļ����������� ��
|
���������������ճ��з�������Ҫ��ѧ��ӦΪ��K2CO3 + H2O + CO2 ==2KHCO3����ֽ���з�Ӧ�Ļ�ѧ����ʽΪ ���������п�ѭ�����õ������� ��
���ںϳ�����������X��H2����Ӧ���ɼ״���CH3OH����ˮ���÷�Ӧ��ѧ��Ӧ����ʽΪ ��������X��CH4������CO2����������4��11����Ӧ���Ӹ��������ԭ�����������ʱ�����ɵ��л��ﻯѧʽΪ ��
��1����ʯȼ�ϵ�ȼ�� ֲ�������á���2����
��3������������
��4����2KHCO3 K2CO3 + H2O + CO2�� K2CO3
K2CO3 + H2O + CO2�� K2CO3
��3H2+CO2=CH3OH+H2O C2H4O2
����������





 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�