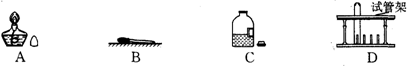
��Ŀ����
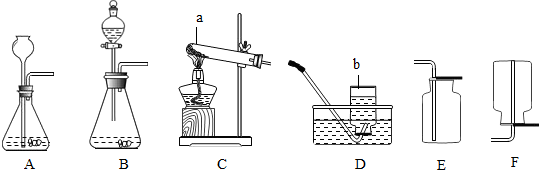
������NH3����һ����ɫ���д̼�����ζ�����壬��������ˮ������ˮ��Һ��Ϊ��ˮ���Լ��ԣ����ڻ�ѧ��ҵ����;�ܹ㷺�������ƻ��ʡ��ƴ���ȣ����������������ڻ�����������1��ʵ�����ռ������ɲ��õķ���Ϊ
��2����ҵ����ϸ���������ð��������״��ķ�ˮ��ʹ���Ϊ����N2��CO2���Ӷ������Ի�������Ⱦ���йصķ�ӦΪ��6NH3+5CH3OH+12B
| ||
��3����400�桢�������ڵ������£��ð����ɽ��ж�����NO��ԭ������N2��H2O��
��д���÷�Ӧ�Ļ�ѧ����ʽ��
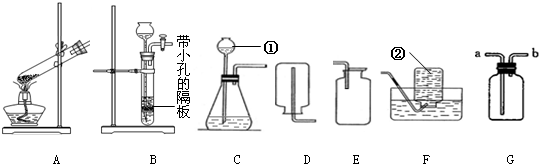
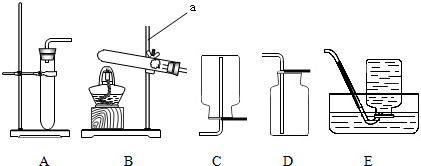
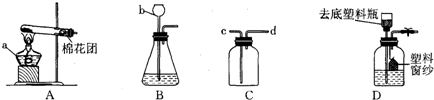
�������������ʵ��������ʿ���ѡ���ռ�����ķ������������ʵĻ�ѧ���ʿ���ѡ�����������������غ㶨�ɿ���ȷ�����ʵĻ�ѧʽ�����ݷ�Ӧ��������������д��ѧ����ʽ��
����⣺��1����Ϊ�������ܶȱȿ���С�����Թ����������ƻ��ʯ�һ���ʯ�ҵȣ���һ�֣��������ſ������ռ��������������Լ��Եĸ�������ﰱ������������ſ�������
��2�����������غ㶨�ɿ�֪��B�к���������ԭ�ӣ������������O2
��3����Ӧ�Ļ�ѧ����ʽΪ��4NH3+6NO
5N2+6H2O
��2�����������غ㶨�ɿ�֪��B�к���������ԭ�ӣ������������O2
��3����Ӧ�Ļ�ѧ����ʽΪ��4NH3+6NO
| ||
| �� |
������������Ҫ������ѡ���ռ�����ķ�����ѡ������������д��ѧ����ʽ�ȷ�������ݣ��������ʹ�ù����Ǽ��Ը�������ܸ����������壬���Ը�������ܸ�����Եģ�������ǿ�����ԵIJ��ܸ��ﻹԭ�Ե����壮

��ϰ��ϵ�д�
 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ