��Ŀ����
 ��ͼ�ǡ������������Ƽ���Ʒ��ǩͼ������ݱ�ǩ��Ϣ����������⣺
��ͼ�ǡ������������Ƽ���Ʒ��ǩͼ������ݱ�ǩ��Ϣ����������⣺
��1��ȱ����Ԫ���������ļ�����________������ţ�
A��ƶѪ��B����״���״�C�����Ͳ���D����Ѫ��
��2��ÿƬҩ�������ٺ���Ԫ�ص�����Ϊ________g��
��3��Ϊ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ��С��ͬѧȡ��4Ƭ���������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥ����40.0g�������ձ���ʣ�����ʵ�������Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ�����������
�����ɶ�����̼������________g��
��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
�⣺��1������ȱ����Ԫ�����������Ͳ���
��2��ÿƬ�к�̼��Ƶ���������Ϊ1.24g����ÿƬҩ�������ٺ���Ԫ�ص�����Ϊ��1.24g���� ��100%��=0.496g��
��100%��=0.496g��
��3������Ϊ̼��ƺ�ϡ���ᷴӦ�ų�������̼�����������غ㶨�ɿ�֪���ձ��е����ʼ��ٵ������������ɵĶ�����̼���������������ɶ�����̼������Ϊ��2.5g��4+40g-47.8g=2.2g��
����4ƬƬ����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 2.2g
 =
=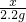
���x=5g��
ÿƬ�к���̼��Ƶ�����Ϊ��5g��4=1.25g��1.24g��
���Ը�Ƭ��̼��Ƶĺ�����ע��ʵ��
�ʴ�Ϊ����1��C����2��0.496��
��3����2.2���ڸ�Ƭ��̼��Ƶĺ�����ע��ʵ��
��������1�����ݸ�Ԫ�ض���������ý��з�����ȱ����Ԫ�����������Ͳ���
��2����Ԫ�ض���̼������ˣ���̼��Ƶ���������Ԫ����̼����е����������������Ԫ�ص�������
��3���ٸ��������غ㶨�ɽ��з������ձ��е����ʼ��ٵ������������ɵĶ�����̼��������
���ɶ�����̼�����������ݷ�Ӧ�ķ���ʽ�����ÿƬ��̼��Ƶ������������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
������������Ҫ������������Ԫ�ص����á����������ļ��㣬���������غ㶨�������Ӧ�ų�������̼���������ǽ��к������Ļ��������ֳ�����֪ʶ���������������
��2��ÿƬ�к�̼��Ƶ���������Ϊ1.24g����ÿƬҩ�������ٺ���Ԫ�ص�����Ϊ��1.24g����
 ��100%��=0.496g��
��100%��=0.496g����3������Ϊ̼��ƺ�ϡ���ᷴӦ�ų�������̼�����������غ㶨�ɿ�֪���ձ��е����ʼ��ٵ������������ɵĶ�����̼���������������ɶ�����̼������Ϊ��2.5g��4+40g-47.8g=2.2g��
����4ƬƬ����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 2.2g
 =
=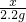
���x=5g��
ÿƬ�к���̼��Ƶ�����Ϊ��5g��4=1.25g��1.24g��
���Ը�Ƭ��̼��Ƶĺ�����ע��ʵ��
�ʴ�Ϊ����1��C����2��0.496��
��3����2.2���ڸ�Ƭ��̼��Ƶĺ�����ע��ʵ��
��������1�����ݸ�Ԫ�ض���������ý��з�����ȱ����Ԫ�����������Ͳ���
��2����Ԫ�ض���̼������ˣ���̼��Ƶ���������Ԫ����̼����е����������������Ԫ�ص�������
��3���ٸ��������غ㶨�ɽ��з������ձ��е����ʼ��ٵ������������ɵĶ�����̼��������
���ɶ�����̼�����������ݷ�Ӧ�ķ���ʽ�����ÿƬ��̼��Ƶ������������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
������������Ҫ������������Ԫ�ص����á����������ļ��㣬���������غ㶨�������Ӧ�ų�������̼���������ǽ��к������Ļ��������ֳ�����֪ʶ���������������

��ϰ��ϵ�д�
 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
�����Ŀ
 ����������ȱ�ƻ�����Ͳ��ͷ�����������ˣ�ÿ�ձ������������ĸƣ���ͼ��ij������Ʒ�IJ���˵������ش�
����������ȱ�ƻ�����Ͳ��ͷ�����������ˣ�ÿ�ձ������������ĸƣ���ͼ��ij������Ʒ�IJ���˵������ش� ��ͼ��ij�ֲ���ҩƷ��ǩ�ϵIJ������֣�ij��ȤС��ͬѧ����������ʵ�飺ȡ��Ƭ��Ƭ�����ȫ��������ƿ�У�Ȼ��80gϡ������Ĵμ��룬ÿ��һ�������ַ�Ӧ������ƿ��ʣ�����������������Ƭ��ֻ��̼����������ᷴӦ���Һ���ˮ��������������̼���ܽ⣩��Ȼ���ټ�ϡ���ᣬ��¼�������±���
��ͼ��ij�ֲ���ҩƷ��ǩ�ϵIJ������֣�ij��ȤС��ͬѧ����������ʵ�飺ȡ��Ƭ��Ƭ�����ȫ��������ƿ�У�Ȼ��80gϡ������Ĵμ��룬ÿ��һ�������ַ�Ӧ������ƿ��ʣ�����������������Ƭ��ֻ��̼����������ᷴӦ���Һ���ˮ��������������̼���ܽ⣩��Ȼ���ټ�ϡ���ᣬ��¼�������±��� ����������ȱ�ƻ�����Ͳ��ͷ�����������ˣ�ÿ�ձ������������ĸƣ���ͼ��ij������Ʒ�IJ���˵������ش�
����������ȱ�ƻ�����Ͳ��ͷ�����������ˣ�ÿ�ձ������������ĸƣ���ͼ��ij������Ʒ�IJ���˵������ش�
