��Ŀ����
��1�������е�ȼ����Ҫ�ɷֿɱ�ʾΪC4H10����д��C4H10��ȫȼ�յĻ�ѧ����ʽ��_____________��
��2���ֻ�.�ʼDZ����Եȵ��Ӳ�Ʒ��ʹ�õ�﮵�ؾ������С���ŵ�ʱ�䳤���ŵ㣬��ŵ�ʱ�Ĺ���ԭ����ﮣ�Li�������������������LiMnO2����д���÷�Ӧ�Ļ�ѧ����ʽ_________________��
��3��6��5�������绷���գ�����������ǡ����������ƥ�����𡱡���������Դ�ḻ����⣨I2���Ļ�ÿɽ����塢���������������õ�NaI��Һ��Cl2�����û���Ӧ���õ��ģ��仯ѧ����ʽΪ___________��
��2���ֻ�.�ʼDZ����Եȵ��Ӳ�Ʒ��ʹ�õ�﮵�ؾ������С���ŵ�ʱ�䳤���ŵ㣬��ŵ�ʱ�Ĺ���ԭ����ﮣ�Li�������������������LiMnO2����д���÷�Ӧ�Ļ�ѧ����ʽ_________________��
��3��6��5�������绷���գ�����������ǡ����������ƥ�����𡱡���������Դ�ḻ����⣨I2���Ļ�ÿɽ����塢���������������õ�NaI��Һ��Cl2�����û���Ӧ���õ��ģ��仯ѧ����ʽΪ___________��
��1��Li+MnO2==LiMnO2
��2��2C4H10+13O2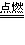 8CO2+10H2O
8CO2+10H2O
��3��Cl2+2NaI==I2+2NaCl
��2��2C4H10+13O2
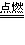 8CO2+10H2O
8CO2+10H2O��3��Cl2+2NaI==I2+2NaCl

��ϰ��ϵ�д�
�����Ŀ