��Ŀ����
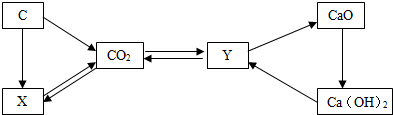
�������������Ľ�������������Ҳ�ڷ������ϱ仯����ͼ�Ǽ��ֳ��õĽ������ϣ�

��1��ͼʾ�Ľ��������У��϶����еĽ���Ԫ���� �����¸�ˮ����ģ�ͱ��������ͬ���ĸֽ��һ������ �仯�����������ѧ������
��2��д����������Ҫ������ʵĻ�ѧʽ��
�������� ��
�ں���Ԫ�صĵ��� ��
��2��ľ�����ڿ�ȼ�Ҫע���������д��һ�����ķ����� ��
��3����ʯ�ҹ��غ�����ˮ����Ƴ�ʯ�ҽ����÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4���ӽ������ϵ�ѡ�����Ǩ����õ�����ʾ�� ��

��1��ͼʾ�Ľ��������У��϶����еĽ���Ԫ����
��2��д����������Ҫ������ʵĻ�ѧʽ��
��������
�ں���Ԫ�صĵ���
��2��ľ�����ڿ�ȼ�Ҫע���������д��һ�����ķ�����
��3����ʯ�ҹ��غ�����ˮ����Ƴ�ʯ�ҽ����÷�Ӧ�Ļ�ѧ����ʽ��
��4���ӽ������ϵ�ѡ�����Ǩ����õ�����ʾ��
���㣺���ʵ�Ԫ�����,��ʯ�ҵ���������;,Ԫ�صļ���,��ѧʽ����д������,��ѧ�仯�������仯���б�,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ,����ԭ���ͷ���
ר�⣺��ѧ����������غ㶨��,��ѧ������
��������1������Ԫ�����ں����д��������Ե�Ԫ�أ����ݱ仯ǰ���Ƿ������������ɿ��ǣ���2������Ҫ����ȷ��д����ѧʽ����2���������ķ������ǣ���3�����ݷ���ʽ��д�����ǣ���4���ӻ�ѧ��������������ÿ��ǣ�
����⣺��1����ʯ���к��и�Ԫ�أ��ֽ��к�����Ԫ�أ����¸�ˮ����ģ�ͱ��������ͬ���ĸֽû�����������ɣ�ֻ����״��״̬�����˱仯�����������仯��
��2����������CH4��
�ں���Ԫ�صĵ���NH4Cl��
��2�����ķ����н����¶ȵ���ȼ���Ż�����£�����ˮ���𣬸��������������ȼ�
��3����Ӧ���������ƺ�ˮ�����������������ƣ����Է���ʽ�ǣ�CaO+H2O=Ca��OH��2��
��4���ӻ�ѧ��������������ÿ��Ǿ��У����绯ѧ�Ը��������ס����������Ҫ���ã�
�ʴ�Ϊ����1���ơ�������������2��CH4��NH4Cl��
��2����ˮ���𣻣�3��CaO+H2O=Ca��OH��2����4����ѧ�ķ�չ�ḻ�����ǵ����
��2����������CH4��
�ں���Ԫ�صĵ���NH4Cl��
��2�����ķ����н����¶ȵ���ȼ���Ż�����£�����ˮ���𣬸��������������ȼ�
��3����Ӧ���������ƺ�ˮ�����������������ƣ����Է���ʽ�ǣ�CaO+H2O=Ca��OH��2��
��4���ӻ�ѧ��������������ÿ��Ǿ��У����绯ѧ�Ը��������ס����������Ҫ���ã�
�ʴ�Ϊ����1���ơ�������������2��CH4��NH4Cl��
��2����ˮ���𣻣�3��CaO+H2O=Ca��OH��2����4����ѧ�ķ�չ�ḻ�����ǵ����
�����������ؼ���Ҫ֪������Ԫ�ص��жϷ�����֪�����ķ�����֪������ʽ����дע�����

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
�����Ԫ�����ڱ���һ���֣������лش���ȷ���ǣ�������
| 11 Na �� | 12 Mg þ | 13 Al �� | 14 Si �� | 15 P �� | 16 S �� | 17 Cl �� | 18 Ar � |
| A��12��Ԫ�ص����ӷ�����Mg+2 |
B����ԭ�ӵĺ�������Ų��� ������ԭ������һ�����Ӵﵽ�ȶ��ṹ ������ԭ������һ�����Ӵﵽ�ȶ��ṹ |
| C����������Ƿǽ���Ԫ�ء��ұ��ǽ���Ԫ�� |
| D����Ԫ�ص����ԭ������Ϊ16 |