��Ŀ����
ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У��������������������������������ͼ��ʾ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش���1����ͼ�п������÷�Ӧ������H2______g��
��2�����������Ļ�ѧ����ʽΪ��______����Ƭ�������ʵ�����Ϊ______g��
��3������������������ռδ����ʱ��Ƭ������������������д��������̣�

���𰸡���������1��ͼ��Ϊ���������������������������仯���������ͼ���֪���ų���������Ϊ1.2g��
��2��δ���������������ᷢ���û���Ӧ�����������Ȼ������������ɷ�Ӧ�ų����������������ݷ�Ӧ�Ļ�ѧ����ʽ���������ᷴӦ����Ƭ��������
��3�����ݲ���������ͼ������50.0gϡ����ʱ��ʼ����Ƭ������Ӧ��ֱ������250.0g����ʱǡ����ȫ��Ӧ��������������Ӧ��������������������ݷ�Ӧ�Ļ�ѧ����ʽ������������������������������Ԫ�ص�����������ñ��������������������ʹ�� ×100%���������������������ռδ����ʱ��Ƭ������������������
×100%���������������������ռδ����ʱ��Ƭ������������������
����⣺��1�����ݲ����������������������������ͼ���ɵ�������������Ϊ1.2g��
�ʴ�Ϊ��1.2��
��2��δ���ʵ��������ᷢ���û���Ӧ�������Ȼ�������������Ӧ�Ļ�ѧ����ʽΪ2Al+6HCl�T2AlCl3+3H2����
����200.0g���ᷴӦ����������Ϊx
2Al+6HCl�T2AlCl3+3H2��
54 219
x 200.0g×21.9%
 =
= x=10.8g
x=10.8g
�ʴ�Ϊ��2Al+6HCl�T2AlCl3+3H2����10.8��
��3������ͼ������������Ӧ�����������Ϊ50.0g����������������Ϊy
Al2O3+6HCl�T2AlCl3+3H2O
102 219
y 50.0g×21.9%
 =
= y=5.1g
y=5.1g
����������������=5.1g× ×100%=2.7g
×100%=2.7g
����������������ռδ����ʱ��Ƭ����������������= ×100%=20%
×100%=20%
�𣺱���������������ռδ����ʱ��Ƭ����������������Ϊ20%��
����������������ĸ��������ڶԷ�Ӧͼ������߽��з�������������ʾ��ʼ����������Ӧ�ų����������ߵ��۵��ʾ��ʱǡ����ȫ��Ӧ��
��2��δ���������������ᷢ���û���Ӧ�����������Ȼ������������ɷ�Ӧ�ų����������������ݷ�Ӧ�Ļ�ѧ����ʽ���������ᷴӦ����Ƭ��������
��3�����ݲ���������ͼ������50.0gϡ����ʱ��ʼ����Ƭ������Ӧ��ֱ������250.0g����ʱǡ����ȫ��Ӧ��������������Ӧ��������������������ݷ�Ӧ�Ļ�ѧ����ʽ������������������������������Ԫ�ص�����������ñ��������������������ʹ��
 ×100%���������������������ռδ����ʱ��Ƭ������������������
×100%���������������������ռδ����ʱ��Ƭ����������������������⣺��1�����ݲ����������������������������ͼ���ɵ�������������Ϊ1.2g��
�ʴ�Ϊ��1.2��
��2��δ���ʵ��������ᷢ���û���Ӧ�������Ȼ�������������Ӧ�Ļ�ѧ����ʽΪ2Al+6HCl�T2AlCl3+3H2����
����200.0g���ᷴӦ����������Ϊx
2Al+6HCl�T2AlCl3+3H2��
54 219
x 200.0g×21.9%
 =
= x=10.8g
x=10.8g�ʴ�Ϊ��2Al+6HCl�T2AlCl3+3H2����10.8��
��3������ͼ������������Ӧ�����������Ϊ50.0g����������������Ϊy
Al2O3+6HCl�T2AlCl3+3H2O
102 219
y 50.0g×21.9%
 =
= y=5.1g
y=5.1g����������������=5.1g×
 ×100%=2.7g
×100%=2.7g����������������ռδ����ʱ��Ƭ����������������=
 ×100%=20%
×100%=20%�𣺱���������������ռδ����ʱ��Ƭ����������������Ϊ20%��
����������������ĸ��������ڶԷ�Ӧͼ������߽��з�������������ʾ��ʼ����������Ӧ�ų����������ߵ��۵��ʾ��ʱǡ����ȫ��Ӧ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
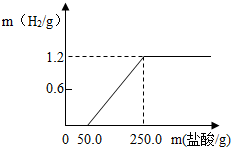 ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У��������������������������������ͼ��ʾ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�
ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У��������������������������������ͼ��ʾ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�