��Ŀ����
��Դ����ʯ�ͻ�����ս��Ŀ�꣬��չ��CH4��CO2��Ϊԭ�ϵġ�C1��ѧ����Ϊ���������ı�Ȼ���ƣ�ͨ����Ȼ���к���H2S���ж����壬��ͼΪ��Ȼ���ϳɰ��Ĺ������̣�
��1������----����ȼ�ϵ���ǽ�______��ת��Ϊ______�ܵ�װ�ã�CH4�ڿ�����ȼ��ʱ����
�����С�ձ����ڻ����Ϸ����ɹ۲쵽�ձ��ڱ���______��
��2 ���ҹ�������Ա���ȷ���һ��������CH4��ֱ�ӷֽ���C6H6��H2����ѧ����ʽΪ
______ 9H2+C6H6
���𰸡�������ȼ�ϵ���ǰѻ�ѧ��ת���ɵ��ܵ�װ�ã����ƶ�����̼�ڿ����к��������߿��Կ�������ЧӦ�����ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ�����������غ㶨�ɿ����ж����ʵĻ�ѧʽ��
����⣺��1������----����ȼ�ϵ���ǽ���ѧ��ת��Ϊ���ܵ�װ�ã������ڿ�����ȼ��ʱ����ˮ�����Խ������С�ձ����ڻ����Ϸ����ɹ۲쵽�ձ��ڱ���ˮ�γ��֣�
��2 ������ɺϳɶ���������л��CH4��ֱ�ӷֽ���C6H6��H2����ѧ����ʽΪ��6CH4�TC6H6+9H2��
��3������CH4��H2O��������Ӧ����CO2��H2����
�ڸ��������غ㶨�ɵ�ʵ�ʿ�֪����������̼����أ�N2�� H2���շ�����1��3��Ӧ����NH3��
��4��ʹ��Fe2O3?H2O��ȥH2S���������к���Ԫ�ص�������2�֣������в���ѭ���������� Fe2O3?H2O��
�ʴ�Ϊ����1����ѧ�����ܣ�ˮ����ˮ�飩
��2��6CH4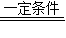 9H2+C6H6
9H2+C6H6
��3����2H2O+CH4�T4H2+CO2��
��KHCO3��3��
��4��2��Fe2O3?H2O��
�����������Ҫ���ջ�ѧ����ʽ����д����������ת���ȷ����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�
����⣺��1������----����ȼ�ϵ���ǽ���ѧ��ת��Ϊ���ܵ�װ�ã������ڿ�����ȼ��ʱ����ˮ�����Խ������С�ձ����ڻ����Ϸ����ɹ۲쵽�ձ��ڱ���ˮ�γ��֣�
��2 ������ɺϳɶ���������л��CH4��ֱ�ӷֽ���C6H6��H2����ѧ����ʽΪ��6CH4�TC6H6+9H2��
��3������CH4��H2O��������Ӧ����CO2��H2����
�ڸ��������غ㶨�ɵ�ʵ�ʿ�֪����������̼����أ�N2�� H2���շ�����1��3��Ӧ����NH3��
��4��ʹ��Fe2O3?H2O��ȥH2S���������к���Ԫ�ص�������2�֣������в���ѭ���������� Fe2O3?H2O��
�ʴ�Ϊ����1����ѧ�����ܣ�ˮ����ˮ�飩
��2��6CH4
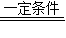 9H2+C6H6
9H2+C6H6��3����2H2O+CH4�T4H2+CO2��
��KHCO3��3��
��4��2��Fe2O3?H2O��
�����������Ҫ���ջ�ѧ����ʽ����д����������ת���ȷ����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ





