��Ŀ����
���ҹ��ƹ�ʹ�õġ����ȼ�ϡ��Ĺ����У�2005���Ϻ���ͨ��ѧ�������ҹ�����ʹ�ö�����Ϊԭ�ϵij��пͳ��������⽫��Ч�Ľ�����������̵����⣮
����֪�����ѵĻ�ѧʽΪC2H6O����ÿ�������ѷ����й�����________��ԭ�ӣ���������̼���⡢������Ԫ�ص�������Ϊ________����д��������ȣ���
�ڶ������ڿ�������ȫȼ�����ɶ�����̼��ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ��________��
�۶�����________��ѡ��ǡ����ǡ������ԡ���ࡱ��ȼ�ϣ�������________��
9 12��3��8 C2H6O+3O2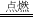 2CO2+3H2O ���� ������ȼ�����ɶ�����̼��������̼������������ЧӦ
2CO2+3H2O ���� ������ȼ�����ɶ�����̼��������̼������������ЧӦ
�������ٸ��ݶ����ѵĻ�ѧʽΪC2H6O���ж�����ӹ��ɺ�Ԫ�ص������Ƚ��н��
�ڸ��ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ���н��
�۸��ݶ�����ȼ��������ˮ�Ͷ�����̼����һ�ֱȽ�������Դ���н��
��𣺢ٸ��ݶ����ѵĻ�ѧʽΪC2H6O����ÿ�������ѷ����й�����2+6+1=9��ԭ�ӣ���������̼���⡢��Ԫ�ص�������Ϊ��12��2������1��6������16��1��=12��3��8��
�ڶ������ڿ�������ȫȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C2H6O+3O2 2CO2+3H2O��
2CO2+3H2O��
�۶�����ȼ��������ˮ�Ͷ�����̼��������̼������������ЧӦ����һ�ֱȽ�������Դ�����Ǿ��Եġ���ࡱ��ȼ�ϣ�
�ʴ�Ϊ����9�� 12��3��8��
��C2H6O+3O2 2CO2+3H2O��
2CO2+3H2O��
�۲��ǣ�������ȼ�����ɶ�����̼��������̼������������ЧӦ��
�����������Ҫ������⻯ѧʽ�ĺ��壬ͬʱҪ���������غ㶨�ɵ����ݣ�ֻ���������ܶ���ط��������������ȷ���жϣ�
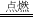 2CO2+3H2O ���� ������ȼ�����ɶ�����̼��������̼������������ЧӦ
2CO2+3H2O ���� ������ȼ�����ɶ�����̼��������̼������������ЧӦ�������ٸ��ݶ����ѵĻ�ѧʽΪC2H6O���ж�����ӹ��ɺ�Ԫ�ص������Ƚ��н��
�ڸ��ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ���н��
�۸��ݶ�����ȼ��������ˮ�Ͷ�����̼����һ�ֱȽ�������Դ���н��
��𣺢ٸ��ݶ����ѵĻ�ѧʽΪC2H6O����ÿ�������ѷ����й�����2+6+1=9��ԭ�ӣ���������̼���⡢��Ԫ�ص�������Ϊ��12��2������1��6������16��1��=12��3��8��
�ڶ������ڿ�������ȫȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C2H6O+3O2
 2CO2+3H2O��
2CO2+3H2O���۶�����ȼ��������ˮ�Ͷ�����̼��������̼������������ЧӦ����һ�ֱȽ�������Դ�����Ǿ��Եġ���ࡱ��ȼ�ϣ�
�ʴ�Ϊ����9�� 12��3��8��
��C2H6O+3O2
 2CO2+3H2O��
2CO2+3H2O���۲��ǣ�������ȼ�����ɶ�����̼��������̼������������ЧӦ��
�����������Ҫ������⻯ѧʽ�ĺ��壬ͬʱҪ���������غ㶨�ɵ����ݣ�ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���������Ļ����ϣ��������֮������ϵ������֪ʶ�����ǻ�ѧѧϰ�е�һ����Ҫ��������ͼ�dz��г�����ѧ����֮������ϵ��

��һ������д��B��C�������ʵ���𣬲���Na��H��O��SԪ����ɵ�����Ϊ������д��һ�ִ������ʵĻ�ѧʽ�������±���Ӧ�Ŀո��
| \ | A | B | C | \ |
| ������� | �� | ______ | ______ | ������ |
| ��ѧʽ | ______ | ______ | ______ | ______ |
���Ϸ�Ӧ��______��
���ֽⷴӦ��______��
�������±��г��˲��������к����϶�Ԫ�ص�ԭ�ӽṹʾ��ͼ����ش��������⣺
| H | O | Na | Mg | Cl | K | Ca |
 |  |  |  |  |  |  |
��2��ԭ�ӵĺ�������Ų����ر��������ĵ�����Ŀ����Ԫ�صĻ�ѧ���������й�ϵ��

��______����______����______��


 ʵ�������ڼ���һ�ִ�����ķ����Ʊ�������
ʵ�������ڼ���һ�ִ�����ķ����Ʊ�������