��Ŀ����
ijУ��ѧ��ȤС���ͬѧ��ʵ������ȡ����ʱ������ȡ����ÿ��п�����涼���Ű�ɫ���壬ѯ����ʦ��֪����ƿп������ʱ�䳤�ˣ��ڱ��汻�����ɰ�ɫ����п���壬����������ͨ��ʵ�����ⶨ��ƿп����Ʒ��п�ı��ʳ̶ȣ��й��������±���
��1����ַ�Ӧ����������������Ϊ g��
��2����ƿп����Ʒ��п������������
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ������ | ����п����Ʒ������ | ����ϡ����������������� | ʣ����������� |
| 10g | 50g | 59.8g | |
��2����ƿп����Ʒ��п������������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�йػ�ѧ����ʽ�ļ���
��������1�����������غ㶨�ɼ��ɽ��
��2����������������������ϻ�ѧ����ʽ�����п�����������ɽ��
��2����������������������ϻ�ѧ����ʽ�����п�����������ɽ��
����⣺��1�����������غ㶨����������������Ϊ��10g+50g-59.8g=0.2g��
��2���裬п����Ʒ��п������Ϊx��
Zn+H2SO4=ZnSO4+H2��
65������������ 2
x��������������0.2g
=
x=6.5g
п����Ʒ��п������������
��100%=65%��
�𣺣�1����������������Ϊ0.2g����2����ƿп����Ʒ��п����������Ϊ65%��
��2���裬п����Ʒ��п������Ϊx��
Zn+H2SO4=ZnSO4+H2��
65������������ 2
x��������������0.2g
| 65 |
| x |
| 2 |
| 0.2g |
x=6.5g
п����Ʒ��п������������
| 6.5g |
| 10g |
�𣺣�1����������������Ϊ0.2g����2����ƿп����Ʒ��п����������Ϊ65%��
����������Ϊ���ݻ�ѧ����ʽ�Ļ������㣬���ʱע�����Ĺ淶����ȷ��д��ѧ����ʽ�������ļ�����̼���ȷ�ļ�������

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
�����Ŀ
����ͼ��ʾ����Ľ��Ͳ���ȷ���ǣ�������
A�� ��ͼ���ʹ�����Ϩ��˵��CO2�Ȳ�ȼ��Ҳ��֧��ȼ�գ����ܶȱȿ����Ĵ� |
B�� ��ͼ���¶ȸߵͶԷ��ӵ������˶�û��Ӱ�� |
C�� ��ͼ��ˮ�ϵİ���ȼ�գ�˵����߱���ȼ�յ��������� |
D��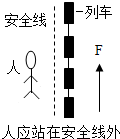 ��ͼ����Ϊ����ѹǿ�Ĵ�С�������йأ�������Ӧվ�ڰ�ȫ���� |
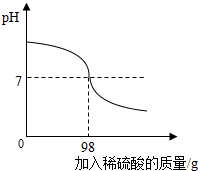 ��ֽ��������������������Ƶķ�ˮ���辭���������Ժ��ŷţ�Ϊ�ⶨ�˷�ˮ���������Ƶ�����������С��ȡ80g��ˮ��Ʒ���뵽��ƿ�У���μ���10%��ϡ���ᣬ��ͼ��ʾ��
��ֽ��������������������Ƶķ�ˮ���辭���������Ժ��ŷţ�Ϊ�ⶨ�˷�ˮ���������Ƶ�����������С��ȡ80g��ˮ��Ʒ���뵽��ƿ�У���μ���10%��ϡ���ᣬ��ͼ��ʾ��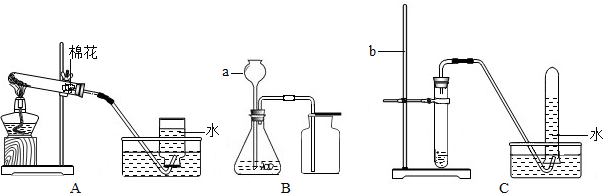
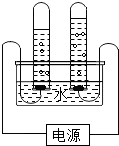 ��ͼ�ǵ��ˮ�ļ���װ��ͼ��
��ͼ�ǵ��ˮ�ļ���װ��ͼ��