��Ŀ����
ˮ������֮Դ������Ӧ���˽�ˮ������ˮ��Դ��
��1������ˮ�Ļ�ѧ���ʵ���С���ǣ��û�ѧ�����
��2������ˮ���������У���ȥˮ�в��������ʿɲ��õķ�����
��3�������ļ��־�ˮ�����У������̶���ߵIJ�����
��4����ˮ���ڻ�ѧʵ���е����ò���С�ӣ�

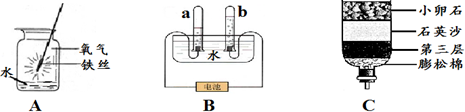
��ش�ʵ��A��ˮ��������
��5�����й���������ˮ��˵���У�����ȷ����
A��������ˮ���ܼ���ˮ B��������һ������������Һ���о�һ�Ե�����
C������������ˮ����Ϊ���岹������
D���Ȼ��Ʒ����DZ����Ȼ��ƻ�ѧ���ʵ���С��
��6����ϸ�������£������ð����������м״���CH3OH���Ĺ�ҵ��ˮ���йط�Ӧ�Ļ�ѧ����ʽΪ
5CH3OH+12O2+6NH3
3X+5CO2+19H2O����X�Ļ�ѧʽΪ
��1������ˮ�Ļ�ѧ���ʵ���С���ǣ��û�ѧ�����
ˮ����
ˮ����
����2������ˮ���������У���ȥˮ�в��������ʿɲ��õķ�����
���������
���������
������̿������������
����
����3�������ļ��־�ˮ�����У������̶���ߵIJ�����
����
����
����4����ˮ���ڻ�ѧʵ���е����ò���С�ӣ�

��ش�ʵ��A��ˮ��������
�������ɵĶ�������
�������ɵĶ�������
��ʵ��B��ˮ�������Ƿ�ֹ���ڵ�������ը��ƿ�ף�д���÷�Ӧ�Ļ�ѧ����ʽ3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
��ʵ��C�е�ˮ���ṩ�����⣬������
| ||
��������
��������
���ã���5�����й���������ˮ��˵���У�����ȷ����
D
D
������ĸ����A��������ˮ���ܼ���ˮ B��������һ������������Һ���о�һ�Ե�����
C������������ˮ����Ϊ���岹������
D���Ȼ��Ʒ����DZ����Ȼ��ƻ�ѧ���ʵ���С��
��6����ϸ�������£������ð����������м״���CH3OH���Ĺ�ҵ��ˮ���йط�Ӧ�Ļ�ѧ����ʽΪ
5CH3OH+12O2+6NH3
| ||
N2
N2
����������1�����ݱ���ˮ�Ļ�ѧ���ʵ���С������ˮ���ӷ�����
��2����������ˮ���������̰���������---����---����---����ǰ�������趼�dz�ȥˮ�в��������ʵģ��������dz�ȥ���������ʵģ����û���̿����ˮ�е���ζ������
��3�����������ܳ�ȥ���е�������ˮ�����ʵõ�������ˮ������
��4���Ƚ���ͼ����ʵ���У�ˮ�����ã�������д����Ӧ�Ļ�ѧ����ʽ��
��5��������Һ�ľ�һ�Ժ��ȶ��Լ���Һ�������ʺ��ܼ���ɵģ�ֻҪ��ˮ��Һ���ܼ���ˮ������
��6�����������غ㶨��ԭ�ӵ��������Ŀ��Ӧǰ�������ı������
��2����������ˮ���������̰���������---����---����---����ǰ�������趼�dz�ȥˮ�в��������ʵģ��������dz�ȥ���������ʵģ����û���̿����ˮ�е���ζ������
��3�����������ܳ�ȥ���е�������ˮ�����ʵõ�������ˮ������
��4���Ƚ���ͼ����ʵ���У�ˮ�����ã�������д����Ӧ�Ļ�ѧ����ʽ��
��5��������Һ�ľ�һ�Ժ��ȶ��Լ���Һ�������ʺ��ܼ���ɵģ�ֻҪ��ˮ��Һ���ܼ���ˮ������
��6�����������غ㶨��ԭ�ӵ��������Ŀ��Ӧǰ�������ı������
����⣺��1������ˮ�Ļ�ѧ���ʵ���С�����ǣ�д���ƣ�ˮ���ӣ�
��2������ˮ���������̰���������---����---����---����ǰ�������趼�dz�ȥˮ�в��������ʵģ��������dz�ȥ���������ʵģ����û���̿����ˮ�е���ζ���ʴ𰸣���������ˣ�������
��3�������ܳ�ȥ���е�������ˮ�����ʵõ�������ˮ���ʴ𰸣�����
��4����ʵ��A����ȼ�����ɶ�����������ˮ���������������ɵĶ�������
��ʵ��B��ˮ�������Ƿ�ֹ������ۻ���ը��ƿ�ף�д���÷�Ӧ�Ļ�ѧ����ʽ3Fe+2O2
Fe3O4��
��ʵ��C�е�ˮ�����ṩ����֮�⣬�����˸����������ã�
��5����Һ�ľ�һ�Ժ��ȶ��Լ���Һ�������ʺ��ܼ���ɵģ�ֻҪ��ˮ��Һ���ܼ���ˮ���ʴ�ѡD��
��6�������غ㶨��ԭ�ӵ��������Ŀ��Ӧǰ�������ı䣬�ʴ𰸣�N2
�ʴ�Ϊ����1��ˮ���ӣ�
��2����������ˣ�������
��3������
��4���������ɵĶ�������ֹ������ۻ���ը��ƿ�ף�3Fe+2O2
Fe3O4������������
��5��D��
��6��N2��
��2������ˮ���������̰���������---����---����---����ǰ�������趼�dz�ȥˮ�в��������ʵģ��������dz�ȥ���������ʵģ����û���̿����ˮ�е���ζ���ʴ𰸣���������ˣ�������
��3�������ܳ�ȥ���е�������ˮ�����ʵõ�������ˮ���ʴ𰸣�����
��4����ʵ��A����ȼ�����ɶ�����������ˮ���������������ɵĶ�������
��ʵ��B��ˮ�������Ƿ�ֹ������ۻ���ը��ƿ�ף�д���÷�Ӧ�Ļ�ѧ����ʽ3Fe+2O2
| ||
��ʵ��C�е�ˮ�����ṩ����֮�⣬�����˸����������ã�
��5����Һ�ľ�һ�Ժ��ȶ��Լ���Һ�������ʺ��ܼ���ɵģ�ֻҪ��ˮ��Һ���ܼ���ˮ���ʴ�ѡD��
��6�������غ㶨��ԭ�ӵ��������Ŀ��Ӧǰ�������ı䣬�ʴ𰸣�N2
�ʴ�Ϊ����1��ˮ���ӣ�
��2����������ˣ�������
��3������
��4���������ɵĶ�������ֹ������ۻ���ը��ƿ�ף�3Fe+2O2
| ||
��5��D��
��6��N2��
�������˽���ӡ�ԭ�ӡ����ӡ�Ԫ��������֮��Ĺ�ϵ���˽⡰���ʵ���ɺͽṹ�����������ʡ��Ĺ۵㣮

��ϰ��ϵ�д�
�����Ŀ
 ˮ������֮Դ��û��ˮ��û��������
ˮ������֮Դ��û��ˮ��û��������
