��Ŀ����
(4��)��ȷ��ʵ������ܱ�֤ʵ��˳�����С��밴Ҫ������������⡣
(1)��ȼ��ȼ������ǰ��һ��Ҫ�� ��
(2)һ����̼��ԭ������ʱ��ͨ��һ����̼�ͼ��ȵ��Ⱥ�˳����____________��
(3)�����Ȼ�����Һʱ���ò��������裬��Ŀ���� ��
(4)ijѧ������Ͳ��ȡҺ�壬��Ͳ�ڷ�ƽ�ȣ���ѧ����Կ̶ȣ����ȸ��Ӷ���Ϊ68mL���㵹����Һ��������Ӷ���Ϊ60mL�����ʡʵ�ʵ�����Һ������Ϊ (�����)��
A������8mL B������8mL C����8mL
���𰸡�
(1) ��������Ĵ���
(2) ��ͨ��CO�����
(3) �����Ȼ����ܽ�
(4) C
����������

��ϰ��ϵ�д�
 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ

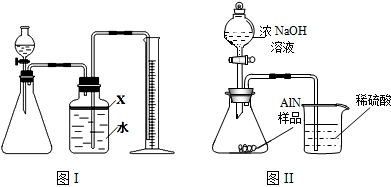
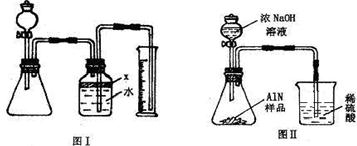

 ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��
ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��