��Ŀ����
��2011��ɽ��������8�⣩���ʵ�������仯
��ʦ�ڿ�������ʾ�����µ�3����Ȥ��ʵ�飺
��������ǯ�г�һС��ʯ��ʯ���þƾ��Ƽ��ȡ�2���Ӻ��ʯ��ʯ���뺬�з�̪��Һ������ˮ�У���̪��Һ����ɫ��
��������ǯ�гָ�ʯ��ʯ�����þƾ��Ƽ��ȣ�ͬʱ�������ͨ��������1���Ӻ�ʯ��ʯ�ٴη��뺬�з�̪������ˮ�У���̪��Һ������ɺ�ɫ
������б�����Һ����εμ����ᣬ��̪��Һ�ֱ�Ϊ��ɫ��
��ش��������⣺
��1������˵������ȷ���ǣ� ��
A��ʯ��ʯ���߱����ȷֽ������
B��ʵ����У������ȵ������£�ʯ��ʯû�з�����ѧ�仯
C����ѧ��Ӧ��Ҫһ������������ʵ����У������¶ȴﲻ��ʯ��ʯ�ֽ���¶�
��2��ͨ����������Ӱ��̼��ƷֽⷴӦ��ԭ���ǣ���ȷ���ǣ� ��
A�������ܽ���̼��Ʒֽ�����Ҫ���¶�
B�������ܸı�̼��Ƶ�����
C��������CaCO3�ֽ�Ĵ���
D�������˵�λʱ���ھƾ������������ӷ�����Ч��ײ�Ĵ���������˵�����¶ȡ�
��3���ڳ��нΣ��漰�����ʵĻ�ѧ������Ҫ�У�
A����ȼ�ԣ�B�����ȶ��ԣ�C�����ԣ�D������;E��������ԣ�F���ǽ������
�������仯�У��ƾ����ֵĻ�ѧ�����ǣ�����ţ���ͬ��__________________;������ֵĻ�ѧ������_____________________��
��4��д������ʵ���з������йط�Ӧ�Ļ�ѧ����ʽ��
_______________________________________________________________________
��1��A ��2��D ��3��A C
��4��(4)CaCO3
 CaO
+CO2 ��;CaO+H2O=Ca(OH)2
CaO
+CO2 ��;CaO+H2O=Ca(OH)2
Ca(OH)2+2HCl =CaCl2+2H2O
; C2H5OH+3O2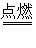 2CO2+3H2O
2CO2+3H2O
����������

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д���������ʳƷ���Ӽ����˹�����ʳƷ��ȫ�뽡����ʳ�Ľ������⣬�����谷�����⾫��Ⱦɫ��ͷ������ѿ�������ݡ������ձ���������Ŀǰ�ҹ��н�90%��ʳƷ�к��кϷ���Υ����ʳƷ���Ӽ�����ô����ο�ѧ�ؿ���ʳƷ���Ӽ���
����֪������ѧ����ʶ���ʡ���ѡ���ʺ�Ӧ�����ʵĿ�ѧ���ӻ�ѧ�ӽǿ�ʳƷ���Ӽ�����Ҫ�Ǵ���Ԫ�������ṹ��������ѧ���ʼ��������ڵı仯�ȷ��棬�±��и�����4��ʳƷ���Ӽ����Իش��й����⣺
(1)�������±��հ״��������ʵĻ�ѧʽ��
| ���ʵ���������� | ������ʳƷ���Ӽ� | |||
| �������� | ������� ���ѧʽ�� | ��Ҫ��ѧ���ʼ��������ڱ仯 | �������� | ���ɹ涨 |
| ̼������ | | ���������ԣ�����θ�ᷴӦ | ���ɼ� | ���� |
| ����ͭ | | | ��ɫ | �����Ͻ�ʹ�� |
| ������ԭ�����ۣ� | | | �������� | ���ݹ��ұ����� |
| ��ȩ | CH2O | | ���� | |
���о�����ʶ���ʵ�����ʱ���ֳ����������ʵ���ɺͽṹ�����ʽ��з��࣬��ԭ������_________________________________��
��3����ԭ����������ʳƷ���Ӽ������ܹ���ֹʳƷ�������������⣬����һ��Ӫ��ǿ������������Ϊ_____________________________

