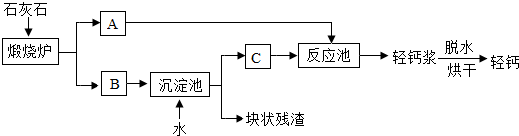
��Ŀ����
����ơ���һ�ֿ�����ϸ�����Ⱥܸߵ�̼��Ʒ�ĩ���й㷺����;��������������Ƭ�����εȡ�����ij��Ƴ��õ��طḻ��ʯ��ʯ��ͨ�����������ơ���ơ���

��1��̼����и�Ԫ�ص�����������_________%��ʳ��������̼��������ڷ�ֹ����ȱ
�������_______________________�����������֢����ƶѪ��������
��2��ʯ��ʯ������ת��ΪA��B���÷�Ӧ����_____________���������Ӧ���ͣ���
��3���������еõ��Ŀ�״�������ܺ���δ����ʯ��ʯ��������Ա��������м��飬�۲쵽 ��֤�������к���ʯ��ʯ��
��4������������Ա���������̼���ƴ��������̼���������Ʒ�Ӧ��������̼��Ƶ�ͬʱ���ɵõ���������(һ����Ҫ�ļ����Ӧ��ԭ����Na2 CO3+Ca(OH)2=CaCO3��+2NaOH����ͨ���������������ַ�������50t̼���ʱ���ܵõ��������Ƶ������Ƕ��٣�
CO3+Ca(OH)2=CaCO3��+2NaOH����ͨ���������������ַ�������50t̼���ʱ���ܵõ��������Ƶ������Ƕ��٣�
��1��40 ��������--------------------����2��,��4�֣�
��2���ֽⷴӦ -------------------------------------------��2�֣�
��3��������ð��------------------------------------------��2�֣�
��4���⣺��õ��������Ƶ�����Ϊ x
Na2CO3+Ca(OH)2=CaCO3��+2NaOH
100 80
50t x ----- ---------------��2�֣�
---------------��2�֣�
100︰80=50t︰x -------------��2�֣�
x =40t ------------------��2�֣�
���ܵõ��������Ƶ�����Ϊ40t��