��Ŀ����
��9�֣����������������������Ӧ��ʮ�ֹ㷺��
1.��������ͭ������㷺ʹ�õ����ֽ��������ʼʹ�������ֽ������Ⱥ�����Ϊ___����Ԫ�ط��ű�ʾ����
2.Ŀǰ���Ǵ���ʹ�õ��ǺϽ�����Ǵ�������������Ϊ�Ͻ���и����������ܣ�����ֱȴ�����Ӳ�� �����С������
3.���������ڿ������γɾ��б������õı�Ĥ�����ijɷ��� ������Ʒ���������⣬�������ȥ����ķ�Ӧ����ʽΪ ���÷�Ӧ�Ļ��������� ��
4.��mg����ͭ����ϡ��������ȫ�ܽ���ټ������۳�ַ�Ӧ�����ˣ��õ�����A����ҺB���ٽ�����A��������ϡ�����У�������ð������ַ�Ӧ��ʣ��������ʵ�����Ϊ3.2g��������A�ijɷ���__________��ԭ����ͭ������m=______________g��
����9�֣�
1. Cu��Fe��Al (1��) ��
2.�� (1��)
3.Al2O3 (1��) ��Fe2O3 + 6HCl=== 2FeCl3+ 3H2O(2��)�����ֽⷴӦ(1��)
4.����ͭ (1��) , (5) 4 (2��) ��
����������1�����ʼʹ�ý������Ⱥ�����ͽ����Ļ��˳���йأ�Խ����Խ��ұ�����������ʼʹ�������ֽ������Ⱥ�����ΪCu��Fe��Al����3��������������Ӧ������������Ĥ��������Ҫ�ɷ�Ϊ�������������ᷴӦ���ڸ��ֽⷴӦ����4��������A��������ϡ�����У�������ð��˵������ʣ�࣬�������ɷ�Ϊ��ͭ������ʣ���3.2g����Ϊͭ�����ݻ�ѧ��Ӧǰ��Ԫ���������䣬����ԭ����ͭ��ͭԪ�ص�����Ҳ��3.2�ˣ�����ԭ����ͭ������m=3.2/(64/80)=4g

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д���30�֣�������Ի�ѧ��
��1�������е�ȼ��
���Ż��Ƚϣ�����ú Сľ�������������������=��������ú¯�����У������������Ŀ���� ��ú����ȫȼ�ղ���һ���ж�����Ļ�ѧʽ�� ��
����ˮ����ԭ���� ������ˮ��Ĥ��ĭ���������������ʱ��������Һ�����ĭ����������չ�γ�һ��ˮĤ���������ԭ���� ��
��2����������������;�㷺
������������Ʒ����Ҫ���ɽ��������Ƴɵ��� ��
�����������ʳ��ʱ������������м��θ��ת��Ϊ�ɱ����յ�Fe2+����Ӧ�Ļ�ѧ����ʽΪ ���� ��Ӧ���������Ӧ���ͣ���
����������װҩ���Ҫ���������� �ԣ�
��ͭ���кܺõ� �ԣ��ʿ������ߡ�����ʪ��ұ���������ָ ���ѧ����ʽ����ͭ�Ϳ����е�O2��H2O�� ������ͭ���Cu2(OH)2CO3�ݣ���Ӧ�Ļ�ѧ����ʽ�� ��
��3�������е�ˮ����Һ
�ٴ�����Ȼˮʱ�����õĻ������� �������ƣ�������������һ�����͵����������仯ѧʽΪ ��
��������500mL 0.9%��������ˮ���ܶ�Ϊ1.0 g/mL������ҪNaCl������Ϊ g�����ƹ����У��������������� ������NaClʱ����������������̣�1 g���������룩��������������ȷ����������Һ���������������� 0.9%�����������������=����
����ͼ�Ǿ�����ˮ�ļ���װ�ã�����˵����ȷ���� ��
| A���������ˮ�Ǵ����� | B����װ���ܶ�ˮɱ������ |
| C����װ���ܰ�Ӳˮ��Ϊ��ˮ | D������̿������ˮ�е�ɫ�ؼ���ζ |
| �¶�/�� | 0 | 20 | 40 | 60 | 80 |
| �ܽ��/g | 13.3 | 31.6 | 63.9 | 110 | 169 |
b��20��ʱ����20gKNO3����50gˮ�г���ܽ⣬������Һ������Ϊ g��
c������60��ʱ��KNO3������Һ210 g��������20�棬������KNO3 g��
��4��ʳƷ��װѧ�ʴ�
�������ز�����˻�����������հ�װ���ӳ���ʳƷ�ı����ڣ�ԭ���� ��
�ڽ�N2�����װ������������������ΪN2�Ļ�ѧ���� ������á����ȶ�������
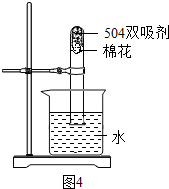
����ͼ�ǡ�504˫�������ı�ǩ�����ʴ��������⣺

a���������ٺ��� �ֵ��ʣ�
b��ȡ����˫������������ˮ�У����ã�����ϲ���Һ��pH=9������pH��ֽ�ⶨ����������� ��ͨ��CO2���ϲ���Һ����ǣ�д�������仯�Ļ�ѧ����ʽ �� ��
c��Ϊ�ⶨ��˫������ʹ��Ч����ȡ����˫������������ͼ��ʾ��ʵ�飬һ��ʱ������Թ���ˮ����� 21%�����������������=������������ ��

d������ʧЧ��˫�����У��к���ɫ���壬����Ҫ�� ��
e����˫�����У�NaCl�����ÿ����� ��
������Ի�ѧ��
��1�������е�ȼ��
���Ż��Ƚϣ�����ú______Сľ�������������������=��������ú¯�����У������������Ŀ����______��ú����ȫȼ�ղ���һ���ж�����Ļ�ѧʽ��______��
����ˮ����ԭ����______������ˮ��Ĥ��ĭ���������������ʱ��������Һ�����ĭ����������չ�γ�һ��ˮĤ���������ԭ����______��
��2����������������;�㷺
����ͼ1����������Ʒ����Ҫ���ɽ��������Ƴɵ���______��
| Ʒ����504˫���� �ɷ֣����ۡ�NaCl��̿��CaO�� |
�����������ʳ��ʱ������������м��θ��ת��Ϊ�ɱ����յ�Fe2+����Ӧ�Ļ�ѧ����ʽΪ______����______��Ӧ���������Ӧ���ͣ���
����������װҩ���Ҫ����������______�ԣ�
��ͭ���кܺõ�______�ԣ��ʿ������ߣ�����ʪ��ұ���������ָ______���ѧ����ʽ����ͭ�Ϳ����е�O2��H2O��______������ͭ��[Cu2��OH��2CO3]����Ӧ�Ļ�ѧ����ʽ��______��
��3�������е�ˮ����Һ
�ٴ�����Ȼˮʱ�����õĻ�������______�������ƣ�������������һ�����͵����������仯ѧʽΪ______��
��������500mL 0.9%��������ˮ���ܶ�Ϊ1.0g/mL������ҪNaCl������Ϊ______g�����ƹ����У���������������______������NaClʱ����������������̣�1g���������룩��������������ȷ����������Һ����������������______0.9%�����������������=����
��ͼ2�Ǿ�����ˮ�ļ���װ�ã�����˵����ȷ����______��
A���������ˮ�Ǵ�������� B����װ���ܶ�ˮɱ������
C����װ���ܰ�Ӳˮ��Ϊ��ˮ�� D������̿������ˮ�е�ɫ�ؼ���ζ
��KNO3������������������Ӫ��Һ���±��ṩ��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȣ�
| �¶�/�� | 0 | 20 | 40 | 60 | 80 |
| �ܽ��/g | 13.3 | 31.6 | 63.9 | 110 | 169 |
b��20��ʱ����20gKNO3����50gˮ�г���ܽ⣬������Һ������Ϊ______g��
c������60��ʱ��KNO3������Һ210g��������20�棬������KNO3______g��
��4��ʳƷ��װѧ�ʴ�
�������ز�--˻�����������հ�װ���ӳ���ʳƷ�ı����ڣ�ԭ����______��
�ڽ�N2�����װ������������������ΪN2�Ļ�ѧ����______������á����ȶ�������
��ͼ3�ǡ�504˫�������ı�ǩ�����ʴ��������⣺
a���������ٺ���______�ֵ��ʣ�
b��ȡ����˫������������ˮ�У����ã�����ϲ���Һ��pH=9������pH��ֽ�ⶨ�����������______��ͨ��CO2���ϲ���Һ����ǣ�д�������仯�Ļ�ѧ����ʽ______��______��
c��Ϊ�ⶨ��˫������ʹ��Ч����ȡ����˫����������ͼ4��ʾ��ʵ�飬һ��ʱ������Թ���ˮ�����______21%�����������������=������������______��
d������ʧЧ��˫�����У��к���ɫ���壬����Ҫ��______��
e����˫�����У�NaCl�����ÿ�����______��
��1�������е�ȼ��
���Ż��Ƚϣ�����ú______Сľ�������������������=��������ú¯�����У������������Ŀ����______��ú����ȫȼ�ղ���һ���ж�����Ļ�ѧʽ��______��
����ˮ����ԭ����______������ˮ��Ĥ��ĭ���������������ʱ��������Һ�����ĭ����������չ�γ�һ��ˮĤ���������ԭ����______��
��2����������������;�㷺
����ͼ1����������Ʒ����Ҫ���ɽ��������Ƴɵ���______��

| Ʒ����504˫���� �ɷ֣����ۡ�NaCl��̿��CaO�� |
�����������ʳ��ʱ������������м��θ��ת��Ϊ�ɱ����յ�Fe2+����Ӧ�Ļ�ѧ����ʽΪ______����______��Ӧ���������Ӧ���ͣ���
����������װҩ���Ҫ����������______�ԣ�
��ͭ���кܺõ�______�ԣ��ʿ������ߣ�����ʪ��ұ���������ָ______���ѧ����ʽ����ͭ�Ϳ����е�O2��H2O��______������ͭ��[Cu2��OH��2CO3]����Ӧ�Ļ�ѧ����ʽ��______��
��3�������е�ˮ����Һ
�ٴ�����Ȼˮʱ�����õĻ�������______�������ƣ�������������һ�����͵����������仯ѧʽΪ______��
��������500mL 0.9%��������ˮ���ܶ�Ϊ1.0g/mL������ҪNaCl������Ϊ______g�����ƹ����У���������������______������NaClʱ����������������̣�1g���������룩��������������ȷ����������Һ����������������______0.9%�����������������=����
��ͼ2�Ǿ�����ˮ�ļ���װ�ã�����˵����ȷ����______��
A���������ˮ�Ǵ����� B����װ���ܶ�ˮɱ������
C����װ���ܰ�Ӳˮ��Ϊ��ˮ D������̿������ˮ�е�ɫ�ؼ���ζ
��KNO3������������������Ӫ��Һ���±��ṩ��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȣ�
| �¶�/�� | 20 | 40 | 60 | 80 | |
| �ܽ��/g | 13.3 | 31.6 | 63.9 | 110 | 169 |
b��20��ʱ����20gKNO3����50gˮ�г���ܽ⣬������Һ������Ϊ______g��
c������60��ʱ��KNO3������Һ210g��������20�棬������KNO3______g��
��4��ʳƷ��װѧ�ʴ�
�������ز�--˻�����������հ�װ���ӳ���ʳƷ�ı����ڣ�ԭ����______��
�ڽ�N2�����װ������������������ΪN2�Ļ�ѧ����______������á����ȶ�������
��ͼ3�ǡ�504˫�������ı�ǩ�����ʴ��������⣺
a���������ٺ���______�ֵ��ʣ�
b��ȡ����˫������������ˮ�У����ã�����ϲ���Һ��pH=9������pH��ֽ�ⶨ�����������______��ͨ��CO2���ϲ���Һ����ǣ�д�������仯�Ļ�ѧ����ʽ______��______��
c��Ϊ�ⶨ��˫������ʹ��Ч����ȡ����˫����������ͼ4��ʾ��ʵ�飬һ��ʱ������Թ���ˮ�����______21%�����������������=������������______��
d������ʧЧ��˫�����У��к���ɫ���壬����Ҫ��______��
e����˫�����У�NaCl�����ÿ�����______��