��Ŀ����
ijѧУ��ѧϰС��Ե��ص�ʯ��ʯ�������е��飬�ⶨʯ��ʯ��̼��Ƶ��������������õķ������£�ȡ��ʯ��ʯ��Ʒ16g����80gϡ������Ĵμ��룬���������������ݼ��±�(��֪ʯ��ʯ��Ʒ�к��еĵ����ʲ�����ˮҲ����ϡ���ᷴӦ)��Ca CO3ʮ2HCl= CaC12ʮH2OʮC02��
����㣺
|
��� |
����ϡ�����������g |
ʣ������������g |
|
��1�� |
20 |
12 |
|
��2�� |
20 |
8 |
|
��3�� |
20 |
4.8 |
|
��4�� |
20 |
n |
(1)�ϱ���n����ֵΪ___________����2 �֣�
��2��ʯ��ʯ��̼��Ƶ���������Ϊ ����6�֣���д��������̣���
(3)��Ӧǰ���������������
(1)
4.8g (2)  =70% (3) 14.6%
=70% (3) 14.6%
�������� (1)����ͼ�����ݿ�֪����1��2�η�Ӧ��������ʼ��ٵ�������Ϊ4g������3�η�Ӧ��������ʼ��ٵ�����Ϊ3.2g��˵����ʱ̼����ѷ�Ӧ�꣬���ٲ������壬�ʱ���n����ֵΪ4.8��
(2) ��Ʒ��̼��Ƶ���������= =70%
=70%
(3) ���������20gϡ����ǡ������ʯ��ʯ�е�4g̼�����ȫ��Ӧ��
�⣺�跴Ӧ�����ĵ����������ΪX��
CaCO3+2HCl==CaCl2+H2O+CO2 ��
100 73
��16-12��g X
100��73=��16-12��g��X
X=73����16-12��g/100=2.92g
�������������Ϊ2.92g/20g��100%=14.6%
���������������Ϊ14.6%��

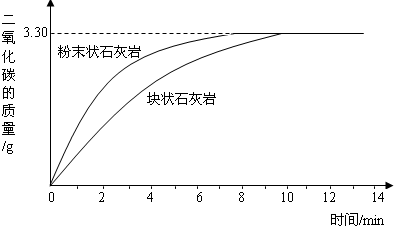
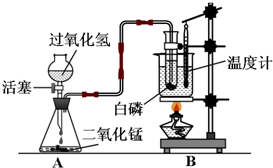 ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮
ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮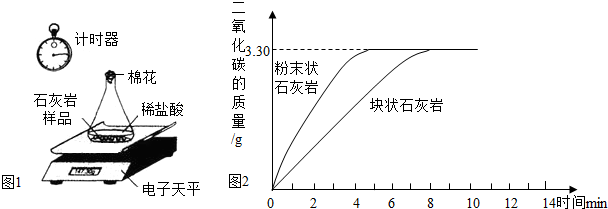
 ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮
ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮