��Ŀ����
ij����ͭ��CuO����ĩ�л�������������ͭ��Cu2O��������һ����С��������ͼ��ʾװ�òⶨ����Cu2O�ĺ����������ͼʾ���ݻش��������⣺
��1��X�dz�����ԭ������CO��H2�е�һ�֣����ܻ�������ˮ�Ͷ�����̼������������װ���ж�X�Ļ�ѧʽΪ��Aװ�õ������ǣ�
��2��Bװ�õ������ǣ���ȱ��������ֱ�ӵ��·�Ӧ��װ���ڵ����ʵ�����ƫ�
��3����֪Cu2O�ڼ��ȵ�������Ҳ�ܱ�X���廹ԭΪCu��д��Cװ�������з�����Ӧ�Ļ�ѧ����ʽ����
��4��Eװ�õ������ǣ�
��5����֪��Ӧǰ������������Ϊ15.2g����ȫ��Ӧ��U�������ʵ�����������2.7����ʧ���Բ��ƣ�����ԭ�������Cu2O������Ϊ��
��6��������C�е����廻�����������е�����һ�֣�ͬ��Ҫ���ø�װ�����Cu2O�����IJⶨʵ�飬��D�е�ҩƷӦ�Ļ�Ϊ��
���𰸡�������������һ���ۺ�ʵ���⣬�������֪��ʵ��Ŀ���Dzⶨ����ͭ��������ͭ�������������ͭ�ĺ�������Ӧԭ�������û�ԭ����������������������ɵ�ˮ���Ȼ������գ�Dװ������������Ϊ���ɵ�ˮ����������֪����ͭ��������ͭ����������������ˮ��������������صĻ�ѧ����ʽ�Ϳ����������ͭ��������ȷ����������京����
������֪�����л���ˮ�����Ͷ�����̼���壬������ȥ�����ˮ�������ͻ���ƫ�Ϊ������������ǰ���뽫���ȥ��������Ũ���ᣬ��������̼������������Һ���ڳ�������̼ʱ�����Һ�ᆳ�����ض�����ˮ����������Ҫ�Ѹ�����������У�
��D�������ͨʱ���Ȼ��ƻ����տ����е�ˮ������ʹ���ˮ����������ƫ����Լ�Eװ�ã����Է�ֹ�����е�ˮ��������Dװ�ã�ʹʵ�����ݸ�ȷ��
����⣺��1��Dװ�õ�����������X������������ɵ����壬��Dװ��ʢ���Ȼ��Ƹ������֪�����յ���ˮ������X����Ϊ��������ѧʽΪ��H2��Aװ����ʢ��Ũ�ռ���Һ�����������̼��Ӧ���������dz�ȥH2�е�CO2���壮
��2��Bװ����ʢ��Ũ���ᣬŨ���������ˮ�ԣ��������Ǹ���H2 ����ȱ����������Я����ˮ��������Cװ��Ҳ�����Dװ�ã����Ȼ������գ�ʹ���ˮ����������ƫ�������ȱ��������ֱ�ӵ��·�Ӧ��Dװ���ڵ����ʵ�����ƫ�
��3����֪Cu2O�ڼ��ȵ�������Ҳ�ܱ�X���廹ԭΪCu��д��Cװ�������з�����Ӧ�Ļ�ѧ����ʽ��
CuO+H2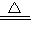 Cu+H2O Cu2O+H2
Cu+H2O Cu2O+H2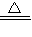 2Cu+H2O
2Cu+H2O
��4����D�������ͨʱ���Ȼ��ƻ����տ����е�ˮ������ʹ���ˮ����������ƫ�����Eװ�õ������ǣ���ֹ������H2O����Dװ���У�
��5����֪��Ӧǰ������������Ϊ15.2g����ȫ��Ӧ��U�������ʵ�����������2.7����ʧ���Բ��ƣ�����ԭ�������Cu2O������Ϊ 7.2g��
��ԭ������� Cu2O������ΪX������H2��Ӧ����ˮ������ΪY����ôCuO������Ϊ��15.2g-X������H2��Ӧ����ˮ������Ϊ2.7g-Y��
CuO+H2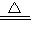 Cu+H2O Cu2O+H2
Cu+H2O Cu2O+H2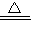 2Cu+H2O
2Cu+H2O
80 18 144 18
15.2g-X 2.7g-y X Y
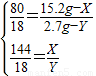
�ⷽ����ã�X=7.2g
����ԭ�������Cu2O������Ϊ7.2g��
��6�����ij�CO����ʱ�����������ɵ�����Ͳ���ˮ�����Ƕ�����̼������Ӧ��D�е�ҩƷӦ�Ļ�Ϊ NaOH��
�ʴ�Ϊ����1��H2����ȥH2��CO2����
��2������H2��D
��3��CuO+H2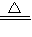 Cu+H2O�� Cu2O+H2
Cu+H2O�� Cu2O+H2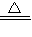 2Cu+H2O
2Cu+H2O
��4����ֹ������H2O����Dװ����
��5��7.2g
��6��NaOH
������������һ���ۺ�ʵ���⣬ʵ��������ܣ������֪ʶ��϶࣬����Ĺؼ�����ȷʵ��Ŀ�ĺ�ԭ���������ۺ�װ�ø����ֵ����ã�
������֪�����л���ˮ�����Ͷ�����̼���壬������ȥ�����ˮ�������ͻ���ƫ�Ϊ������������ǰ���뽫���ȥ��������Ũ���ᣬ��������̼������������Һ���ڳ�������̼ʱ�����Һ�ᆳ�����ض�����ˮ����������Ҫ�Ѹ�����������У�
��D�������ͨʱ���Ȼ��ƻ����տ����е�ˮ������ʹ���ˮ����������ƫ����Լ�Eװ�ã����Է�ֹ�����е�ˮ��������Dװ�ã�ʹʵ�����ݸ�ȷ��
����⣺��1��Dװ�õ�����������X������������ɵ����壬��Dװ��ʢ���Ȼ��Ƹ������֪�����յ���ˮ������X����Ϊ��������ѧʽΪ��H2��Aװ����ʢ��Ũ�ռ���Һ�����������̼��Ӧ���������dz�ȥH2�е�CO2���壮
��2��Bװ����ʢ��Ũ���ᣬŨ���������ˮ�ԣ��������Ǹ���H2 ����ȱ����������Я����ˮ��������Cװ��Ҳ�����Dװ�ã����Ȼ������գ�ʹ���ˮ����������ƫ�������ȱ��������ֱ�ӵ��·�Ӧ��Dװ���ڵ����ʵ�����ƫ�
��3����֪Cu2O�ڼ��ȵ�������Ҳ�ܱ�X���廹ԭΪCu��д��Cװ�������з�����Ӧ�Ļ�ѧ����ʽ��
CuO+H2
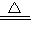 Cu+H2O Cu2O+H2
Cu+H2O Cu2O+H2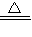 2Cu+H2O
2Cu+H2O��4����D�������ͨʱ���Ȼ��ƻ����տ����е�ˮ������ʹ���ˮ����������ƫ�����Eװ�õ������ǣ���ֹ������H2O����Dװ���У�
��5����֪��Ӧǰ������������Ϊ15.2g����ȫ��Ӧ��U�������ʵ�����������2.7����ʧ���Բ��ƣ�����ԭ�������Cu2O������Ϊ 7.2g��
��ԭ������� Cu2O������ΪX������H2��Ӧ����ˮ������ΪY����ôCuO������Ϊ��15.2g-X������H2��Ӧ����ˮ������Ϊ2.7g-Y��
CuO+H2
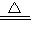 Cu+H2O Cu2O+H2
Cu+H2O Cu2O+H2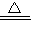 2Cu+H2O
2Cu+H2O80 18 144 18
15.2g-X 2.7g-y X Y
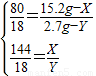
�ⷽ����ã�X=7.2g
����ԭ�������Cu2O������Ϊ7.2g��
��6�����ij�CO����ʱ�����������ɵ�����Ͳ���ˮ�����Ƕ�����̼������Ӧ��D�е�ҩƷӦ�Ļ�Ϊ NaOH��
�ʴ�Ϊ����1��H2����ȥH2��CO2����
��2������H2��D
��3��CuO+H2
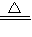 Cu+H2O�� Cu2O+H2
Cu+H2O�� Cu2O+H2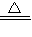 2Cu+H2O
2Cu+H2O��4����ֹ������H2O����Dװ����
��5��7.2g
��6��NaOH
������������һ���ۺ�ʵ���⣬ʵ��������ܣ������֪ʶ��϶࣬����Ĺؼ�����ȷʵ��Ŀ�ĺ�ԭ���������ۺ�װ�ø����ֵ����ã�

��ϰ��ϵ�д�
�����Ŀ
