��Ŀ����
������±��ش��й����⣮
��1��д���ϱ��л��Ļ�ѧʽΪ�� ����ʯ�ҵĻ�ѧʽΪ�� ��
��2���ϱ���������������� �����ڼ���� ������ţ�
��3���������ʸ��������������ϱ��ظ���д��ѧʽ��� �� ��� �� ��
��4�����ϱ��е�ij���ʳ�����Ļ�ѧ��Ӧ����ʽ�� �������������ʱ����ڸ������У�������Ӧ�Ļ�ѧ����ʽΪ�� ��
| ��� | �� | �� | �� | �� |
| ���� | ��ʯ�� | ��� | ���� | ���� |
| ��ѧʽ | Na2CO3 | HCl |
��2���ϱ����������������
��3���������ʸ��������������ϱ��ظ���д��ѧʽ���
��4�����ϱ��е�ij���ʳ�����Ļ�ѧ��Ӧ����ʽ��
���㣺��ѧʽ����д������,������������ᡢ����ε��б�,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧ����������غ㶨��,���ʵķ���
��������1����ȷ���ʵ��׳ƣ����ݻ����ﻯѧʽ����дһ����ɣ�������ǰ���ǽ����ں������������ں�ԭ�Ӹ�������©���������ϼ۴�����Ϊ�������д��
��2������������ͼ�Ķ�����н��
��3�����ݳ����������н��
��4��������������ŵ�������ϡ�����У�����������������ᷴӦ�������ⷴӦ��������������ᷴӦ���������Ҫ�ɷ�Ϊ�����������ݴ˻ش����⼴�ɣ�
��2������������ͼ�Ķ�����н��
��3�����ݳ����������н��
��4��������������ŵ�������ϡ�����У�����������������ᷴӦ�������ⷴӦ��������������ᷴӦ���������Ҫ�ɷ�Ϊ�����������ݴ˻ش����⼴�ɣ�
����⣺��1��������������Ƶ��׳ƣ�������Ԫ����+1�ۣ���������-1�ۣ��ʴ�Ϊ��NaOH����ʯ���������Ƶ��׳ƣ����и�Ԫ����+2�ۣ���Ԫ����-2�ۣ��ʴ�Ϊ��CaO��
��2����������ָ������Ԫ����ɣ�����һ��Ϊ��Ԫ�صĻ�����ʢ���������������ɽ������Ӻ����������ɵģ��ɴ˿�֪�����ڼ���Ǣڣ�
��3������������⣬�������ỹ����������ᣬ�仯ѧʽ�ֱ�Ϊ��H2SO4��HNO3�������ļ����������ƺ����������ȣ��仯ѧʽ
�ֱ�Ϊ��Ca��OH��2��Al��OH��3��
��4���������Ҫ�ɷ�Ϊ����������������ϡ�������䷴Ӧ�Ӷ������⣬��Ӧ�Ļ�ѧ����ʽΪ��6HCl+Fe2O3�T2FeCl3+3H2O�������ⷴӦ������������������Ӧ������ʽΪ��Fe+2HCl=FeCl2+H2����
�ʴ�Ϊ����1��NaOH��CaO����2���١��ڣ���3��H2SO4��HNO3��Ca��OH��2��Al��OH��3����4��Fe+2HCl=FeCl2+H2����Fe+2HCl=FeCl2+H2����
��2����������ָ������Ԫ����ɣ�����һ��Ϊ��Ԫ�صĻ�����ʢ���������������ɽ������Ӻ����������ɵģ��ɴ˿�֪�����ڼ���Ǣڣ�
��3������������⣬�������ỹ����������ᣬ�仯ѧʽ�ֱ�Ϊ��H2SO4��HNO3�������ļ����������ƺ����������ȣ��仯ѧʽ
�ֱ�Ϊ��Ca��OH��2��Al��OH��3��
��4���������Ҫ�ɷ�Ϊ����������������ϡ�������䷴Ӧ�Ӷ������⣬��Ӧ�Ļ�ѧ����ʽΪ��6HCl+Fe2O3�T2FeCl3+3H2O�������ⷴӦ������������������Ӧ������ʽΪ��Fe+2HCl=FeCl2+H2����
�ʴ�Ϊ����1��NaOH��CaO����2���١��ڣ���3��H2SO4��HNO3��Ca��OH��2��Al��OH��3����4��Fe+2HCl=FeCl2+H2����Fe+2HCl=FeCl2+H2����
������������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ȫ�棬ע�ػ�������Ŀ�ѶȽ��ף��ڽ�����ʽ����д��ʱ������ȷ����Ӧԭ����Ȼ��������ԭ���ҳ���Ӧ�������ͷ�Ӧ���������ݷ���ʽ����д������д����ʽ��

��ϰ��ϵ�д�
�����Ŀ
����ʵ�����û�а�ȫ�������ǣ�������
| A����ȼ�ŵľƾ��������Ӿƾ� |
| B������ʱ���㵹Һ���ò��������� |
| C�����ǿ�ֱ�Ӵյ�����ƿ�����������ζ |
| D����ֱ�����Թ���ֱ�ӷ������� |
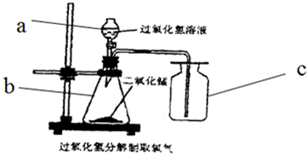 ʵ�����У������÷ֽ����������Һ����������������������ȸ�����صķ�����ȡ������
ʵ�����У������÷ֽ����������Һ����������������������ȸ�����صķ�����ȡ������