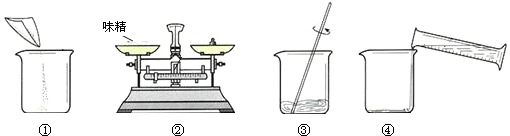
��Ŀ����
31��ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na��������ˮ����AgNO3����Ӧ�����������NaCl�������ɷֲ����ǣ�����ش��������⣺
��1����ȡ5.0gζ�����Ƴ�50g��Һ����ȡ����ˮ����Ͳ�����
A��10mL B��50mL C��100
��2����ͼ�����ƹ��̣���ȷ�IJ���˳��Ϊ

��3��Ϊ�ⶨζ����NaCl��������������������ʵ�飺
���������Ƶ�50g��Һ�м��������AgNO3��Һ��ַ�Ӧ����Ӧ����ʽΪ
�ڹ��˺�ϴ�ӡ��������AgCl���壮
����������������ȷ������£�����������Һ�����У����ӿ̶���ȡ����ˮ������������Һ�ĹȰ�������������
��1����ȡ5.0gζ�����Ƴ�50g��Һ����ȡ����ˮ����Ͳ�����
B
��������ţ�A��10mL B��50mL C��100
��2����ͼ�����ƹ��̣���ȷ�IJ���˳��Ϊ
��
����
����
����
�� ������ţ�1�֣�
��3��Ϊ�ⶨζ����NaCl��������������������ʵ�飺
���������Ƶ�50g��Һ�м��������AgNO3��Һ��ַ�Ӧ����Ӧ����ʽΪ
AgNO3+NaCl�TAgCl��+NaNO3
����������Ƿ���ȫ�ķ����ǣ����ú����ϲ���Һ�м���AgNO3
��Һ���ѧʽ�����۲��Ƿ��г������ɣ��ڹ��˺�ϴ�ӡ��������AgCl���壮
����������������ȷ������£�����������Һ�����У����ӿ̶���ȡ����ˮ������������Һ�ĹȰ�������������
ƫС
���ƫ����ƫС������Ӱ�족������������1��������Һ����=��������+�ܼ���������5.0gζ�����Ƴ�50g��Һ���������ʱ����ˮ������Ȼ��ѡ����ȡ����ˮ����Ͳ��
��2������ʹ�ù�������������Һ�IJ��裺����-����-�ܽ⣬�ж�ʵ�����ͼ�в�����˳��
��3������Һ�е��Ȼ�������������Һ�ܷ�����ѧ��Ӧ�����ɰ�ɫ�������ɲ�ȡ�ٵμ���������Һ�ķ������۲��Ƿ��г�����������˵���Ѿ�������ȫ��
�����Ӷ���ʱ�������������С��ʵ��Һ��������
��2������ʹ�ù�������������Һ�IJ��裺����-����-�ܽ⣬�ж�ʵ�����ͼ�в�����˳��
��3������Һ�е��Ȼ�������������Һ�ܷ�����ѧ��Ӧ�����ɰ�ɫ�������ɲ�ȡ�ٵμ���������Һ�ķ������۲��Ƿ��г�����������˵���Ѿ�������ȫ��
�����Ӷ���ʱ�������������С��ʵ��Һ��������
����⣺��1��ȡ5.0gζ�����Ƴ�50g��Һ������Ҫˮ������=50g-5g=45g��45mL�������Ҫѡ��50mL����Ͳ����ѡB��
��2��ͼ��Ϊ������ϵ�ζ�������ձ���ͼ��Ϊ����ζ����������ͼ��Ϊ�����ܽ⣬ͼ��Ϊ��ȡˮ����ʢ��ζ�����ձ�����ˣ���ȷ�IJ���˳��Ϊ�ڢ٢ܢۣ�
��3�����Ȼ�������������Һ�ܷ�����ѧ��Ӧ�����ɰ�ɫ��������Ӧ��ѧ����ʽΪAgNO3+NaCl�TAgCl��+NaNO3������Һ���ٵμ���������Һ�����۲쵽����������˵��ԭ���Ѿ���ȫ������
������ʵ����ȡˮ����������Ӷ���������ʱ����Ҫˮ���������ˣ�ʹ�����Ƶ���Һ��ˮ��ƫ�����������ƫС��
������1��B
��2��2 1 4 3
��3����AgNO3+NaCl�TAgCl��+NaNO3��AgNO3����ƫС
��2��ͼ��Ϊ������ϵ�ζ�������ձ���ͼ��Ϊ����ζ����������ͼ��Ϊ�����ܽ⣬ͼ��Ϊ��ȡˮ����ʢ��ζ�����ձ�����ˣ���ȷ�IJ���˳��Ϊ�ڢ٢ܢۣ�
��3�����Ȼ�������������Һ�ܷ�����ѧ��Ӧ�����ɰ�ɫ��������Ӧ��ѧ����ʽΪAgNO3+NaCl�TAgCl��+NaNO3������Һ���ٵμ���������Һ�����۲쵽����������˵��ԭ���Ѿ���ȫ������
������ʵ����ȡˮ����������Ӷ���������ʱ����Ҫˮ���������ˣ�ʹ�����Ƶ���Һ��ˮ��ƫ�����������ƫС��
������1��B
��2��2 1 4 3
��3����AgNO3+NaCl�TAgCl��+NaNO3��AgNO3����ƫС
������������нϴ���ۺ��ԣ��ڽ��ʱ��Ҫ�漰�϶�Ļ���֪ʶ����ˣ���Ҫ�㹻�����ĺ�������

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
�����Ŀ
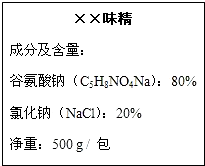 ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�
ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�