��Ŀ����
 ��Դ�ͻ����ǵ����������ٵ��������⣮Ŀǰ����ʯȼ���������������������Ҫ��Դ���ҹ���Ȼ���Ĵ����ḻ������������ʹ���ǹ������ٵĹ�ͬ���⣮
��Դ�ͻ����ǵ����������ٵ��������⣮Ŀǰ����ʯȼ���������������������Ҫ��Դ���ҹ���Ȼ���Ĵ����ḻ������������ʹ���ǹ������ٵĹ�ͬ���⣮��1�������������ǻ���������еĽ�ͷ���֣����ٹ������ͳ����ӡ�С�CNG���ı�־��������������ѹ����Ȼ��Ϊȼ�ϵ�������
����Ȼ������Ҫ�ɷ��Ǽ���--CH4������̼Ԫ�صĻ��ϼ�Ϊ
-4
-4
����ʹ����Ȼ����ȼ���ܷ��������ЧӦ�ķ�����
����
����
����ܡ����ܡ����������ǣ����鱾�������������壬��ȼ�յIJ��������̼Ҳ����������
���鱾�������������壬��ȼ�յIJ��������̼Ҳ����������
����2�����������䡱�����ѻݼ�ǧ����ԭ����Һ��ʯ������ú��Ϊȼ�ϵļ�ͥ����������Ȼ����ȼ�ϣ���Ȼ����Һ��ʯ����ȼ�յķ�Ӧ�ɱ�ʾΪ�ǣ�CH4+2O2
| ||
| ||
��С��������һ����Һ��ʯ����Ϊȼ�ϵ���ߣ���Ҫ������Ȼ����ȼ�ϣ�Ӧ���õĴ�ʩ�ǣ�ע��ͬ�¡�ͬѹ�£���ͬ����IJ�ͬ���������ͬ�ķ�������
A
A
A�����ٿ����Ľ�����������Һ�����Ľ����� B���������ߵĽ�����
C��������������������Һ�����Ľ����� D���������ߵĽ�����
��ȼ��й©�����Σ�գ�ȼ����ȫ�Ǽ�ͥ�����ͷ�ȴ��£�
a��Ϊ�˷�ֹȼ����ɢ������ȼ���м�����������������ζ������C2H5SH���������ȼ��ʱ����������̼�����������ˮ������д�����ȼ�յĻ�ѧ��Ӧ����ʽ��
2C2H5SH+9O2
4CO2+2X+6H2O
| ||
2C2H5SH+9O2
4CO2+2X+6H2O
��
| ||
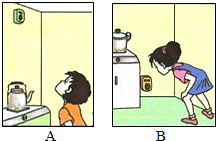
b���ڼ��а�װ�������Ƿ�ֹȼ����ɢ����һ��ʩ�����������Ӵ���һ������й©����ʱ���ᷢ����������λͬѧ������ʹ�õ�ȼ������Ȼ�������жϱ�������װ��λ��Ӧ��ͼ��
A
A
����A��B����ʾ�����жϵ�������������ܶ�С�ڿ���
������ܶ�С�ڿ���
����3����Ȼ������Ҫ�ɷ�����ú��Ŀ���ﵽһ����Ũ�ȣ�������ͻᷢ����ը������Ϊ��˹��ը��Ϊ�˷�ֹ��˹��ը��ú��Ŀ��ڱ����ȡ�İ�ȫ��ʩ��
����ͨ�磨�����������ñ���װ�õȣ�
����ͨ�磨�����������ñ���װ�õȣ�
����������1�����ݻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0�Լ����鱾�������������ȼ�����ɶ�����̼Ҳ������������н��
��2��������Ȼ����Һ��ʯ����ȼ�յĻ�ѧ����ʽ��1���ӵ���Ȼ��ȼ����Ҫ2������������1����Һ��ʯ����ȼ����Ҫ5�����������ɴ˿ɵ�֪��ͬ�����Һ��ʯ������ȫȼ����Ҫ�������������������ͬ�������Ȼ�����Ӷ��жϸ�ΪҺ��ʯ����ʱΪ��֤������ȫȼ�ն��������Ҫ���ĸĶ��������ȼ��ʱ����������̼�����������ˮ�Լ�������ܶ�С�ڿ������ܶȽ��н��
��3�����ݼ������ڿ�ȼ�����壬��������ܻᷢ����ը���н��
��2��������Ȼ����Һ��ʯ����ȼ�յĻ�ѧ����ʽ��1���ӵ���Ȼ��ȼ����Ҫ2������������1����Һ��ʯ����ȼ����Ҫ5�����������ɴ˿ɵ�֪��ͬ�����Һ��ʯ������ȫȼ����Ҫ�������������������ͬ�������Ȼ�����Ӷ��жϸ�ΪҺ��ʯ����ʱΪ��֤������ȫȼ�ն��������Ҫ���ĸĶ��������ȼ��ʱ����������̼�����������ˮ�Լ�������ܶ�С�ڿ������ܶȽ��н��
��3�����ݼ������ڿ�ȼ�����壬��������ܻᷢ����ը���н��
����⣺��1���ٸ��ݻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0�Լ���Ԫ�صĻ��ϼ�Ϊ+1�ۣ�����̼Ԫ�صĻ��ϼ�Ϊ-4�ۣ����-4��
�ڼ��鱾�������������Լ�����ȼ�����ɶ�����̼Ҳ���������壬����ʹ����Ȼ����ȼ�ϲ��ܱ�������ЧӦ�ķ�����������ܣ����鱾�������������壬��ȼ�յIJ��������̼Ҳ���������壻
��2������ͬ����£����������ͬ������������ͬ�����ݷ�Ӧ�Ļ�ѧ����ʽ����ͬ���Ӽ�ͬ�������Ȼ����Һ��ʯ������ȫȼ�գ�Һ��ʯ������Ҫ�����������Ϊȷ��������ȫȼ������Ҫ����������֣���Ҫ���ٿ����Ľ�����������Һ�����Ľ���������ѡA��
��a�������ȼ��ʱ����������̼�����������ˮ�����ȼ�յĻ�ѧ��Ӧ����ʽΪ2C2H5SH+9O2
4CO2+2X+6H2O�����2C2H5SH+9O2
4CO2+2X+6H2O��
b��������ܶ�С�ڿ������ܶȣ����Ա�������װ��λ��Ӧ��ͼ��A��ʾ�����A��������ܶ�С�ڿ�����
��3���������ڿ�ȼ�����壬��������ܻᷢ����ը������Ϊ�˷�ֹ��˹��ը��ú��Ŀ��ڱ����ȡ�İ�ȫ��ʩ�DZ���ͨ�磨�����������ñ���װ�õȣ����������ͨ�磨�����������ñ���װ�õȣ���
�ڼ��鱾�������������Լ�����ȼ�����ɶ�����̼Ҳ���������壬����ʹ����Ȼ����ȼ�ϲ��ܱ�������ЧӦ�ķ�����������ܣ����鱾�������������壬��ȼ�յIJ��������̼Ҳ���������壻
��2������ͬ����£����������ͬ������������ͬ�����ݷ�Ӧ�Ļ�ѧ����ʽ����ͬ���Ӽ�ͬ�������Ȼ����Һ��ʯ������ȫȼ�գ�Һ��ʯ������Ҫ�����������Ϊȷ��������ȫȼ������Ҫ����������֣���Ҫ���ٿ����Ľ�����������Һ�����Ľ���������ѡA��
��a�������ȼ��ʱ����������̼�����������ˮ�����ȼ�յĻ�ѧ��Ӧ����ʽΪ2C2H5SH+9O2
| ||
| ||
b��������ܶ�С�ڿ������ܶȣ����Ա�������װ��λ��Ӧ��ͼ��A��ʾ�����A��������ܶ�С�ڿ�����
��3���������ڿ�ȼ�����壬��������ܻᷢ����ը������Ϊ�˷�ֹ��˹��ը��ú��Ŀ��ڱ����ȡ�İ�ȫ��ʩ�DZ���ͨ�磨�����������ñ���װ�õȣ����������ͨ�磨�����������ñ���װ�õȣ���
�����������������й���Դ������ͻ������⣬��Լ��ʯ��Դ�������Ŀ�������Դ�ǿ�ѧ���������о����¿��⣬�й���Դ����Ҳ�ǽ������п����ȵ�֮һ��ͬѧ��Ҫ������գ�

��ϰ��ϵ�д�
 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
�����Ŀ
 ��2006?����Դ�ͻ����ǵ����������ٵ��������⣮Ŀǰ����ʯȼ���������������������Ҫ��Դ������ȫ����Դʹ����������������ѧʹ�ã���ʯȼ�ϵȲ���������Դ������ݽߣ����Ի�����������Ӱ�죮�������Ҫ�����ǿ������ܡ�̫���ܵ�����Դ��
��2006?����Դ�ͻ����ǵ����������ٵ��������⣮Ŀǰ����ʯȼ���������������������Ҫ��Դ������ȫ����Դʹ����������������ѧʹ�ã���ʯȼ�ϵȲ���������Դ������ݽߣ����Ի�����������Ӱ�죮�������Ҫ�����ǿ������ܡ�̫���ܵ�����Դ��