��Ŀ����
��ҵ�ϡ����ϡ��Ƽ�ƵõĴ����Ʒ�г�����������NaCl��Ϊ�ⶨ�����Ʒ��Na2CO3�ĺ�����ȡ23g��Ʒ�����ձ��У���ˮ�����ܽ⣬Ȼ����ε���ϡ���ᣬ������200gϡ����ʱ������ǡ����ȫ��Ӧ�����ɵ�����ȫ���ݳ������ռ���8.8gCO2��
(1)����ϡ���������ʵ���������Ϊ____________��23g��Ʒ��Na2CO3������Ϊ__________g
(2)��Ӧ���ձ��е���ҺΪ��������Һ����ͨ�������������Һ�����ʵ�������
(1)����ϡ���������ʵ���������Ϊ____________��23g��Ʒ��Na2CO3������Ϊ__________g
(2)��Ӧ���ձ��е���ҺΪ��������Һ����ͨ�������������Һ�����ʵ�������
(1)7.3����21.2
(2)��Ӧ��������Һ������ΪNaCl��������NaCl������Ϊx��
Na2CO3 + 2HCl = 2NaCl + CO2�� + H2O
117 44
x 8.8g
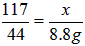
���x=23.4g
NaCl������=23.4g+(23g-21.2g)=25.2g
(2)��Ӧ��������Һ������ΪNaCl��������NaCl������Ϊx��
Na2CO3 + 2HCl = 2NaCl + CO2�� + H2O
117 44
x 8.8g
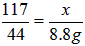
���x=23.4g
NaCl������=23.4g+(23g-21.2g)=25.2g

��ϰ��ϵ�д�
�����Ŀ