��Ŀ����
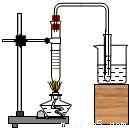
��ͼΪ����������ˮ����ʵ�飬��ش��������⣺
��1��ʵ�鿪ʼӦ����________��
��2�����Թ��м���5mL-l0mL��ˮ������ƿ������Ȼ����С�Թ�������ˮ�м�����������Һ���۲쵽������Ϊ________��
��3���������ַ�������Ч�����
A�������ܼӳ�������B�����ձ��ڼӱ��顡������ C��ʹ��������
��4��ʵ�����ʱӦ����________��������ܡ���Ϩ��ƾ��ơ�������������Ŀ����________
��5��ijͬѧ��ˮ�������������Һ���Ա�ʵ�飬�����а�ɫ�����������÷�Ӧ�Ļ�ѧ����ʽ________��
�⣺��1��ʵ�鿪ʼ������Ӧ�ü��װ�������ԣ���ֹ©������2�����Թ��м���5mL-l0mL��ˮ������ƿ������Ȼ����С�Թ�������ˮ�м�����������Һ���۲쵽������Ϊ�ް�ɫ��������������ˮ��û���������ˣ���3��ʹ��������������Ч����ã���ѡC����4��ʵ�����ʱӦ���ȳ����ܣ���ֹˮ����������Σ�գ���5��ijͬѧ��ˮ�������������Һ���Ա�ʵ�飬�����а�ɫ�����������÷�Ӧ�Ļ�ѧ����ʽ��AgNO3+NaCl=AgCl��+NaNO3����ˮ�����Ȼ��ƣ�
�ʴ�Ϊ��
��1���ȼ��װ�������ԣ�
��2���ް�ɫ����������
��3��C��
��4�������ܷ�ֹˮ����
��5��AgNO3+NaCl=AgCl��+NaNO3
��������1��ʵ�鿪ʼ������Ӧ�ü��װ�������ԣ�
��2��ʵ�鿪ʼ����С�Թ�������ˮ�м�����������Һ���ް�ɫ��������������ˮ�Ǵ���ˮ��
��3��ʹ��������������Ч����ã���ѡC��
��4��ʵ�����ʱӦ���ȳ����ܣ���ֹˮ������
��5��ijͬѧ��ˮ�������������Һ���Ա�ʵ�飬�а�ɫ�����������÷�Ӧ�Ļ�ѧ����ʽ��AgNO3+NaCl=AgCl��+NaNO3����ˮ�����Ȼ��ƣ�
���������⿼��Ժ�ˮ�ĺ�������������������ˮ���Ǻ�ˮ����Ҫ����֮һ����ԭ����ʹ��ˮ������������ʹ�����������Ӷ��õ���ˮ�ķ�����
�ʴ�Ϊ��
��1���ȼ��װ�������ԣ�
��2���ް�ɫ����������
��3��C��
��4�������ܷ�ֹˮ����
��5��AgNO3+NaCl=AgCl��+NaNO3
��������1��ʵ�鿪ʼ������Ӧ�ü��װ�������ԣ�
��2��ʵ�鿪ʼ����С�Թ�������ˮ�м�����������Һ���ް�ɫ��������������ˮ�Ǵ���ˮ��
��3��ʹ��������������Ч����ã���ѡC��
��4��ʵ�����ʱӦ���ȳ����ܣ���ֹˮ������
��5��ijͬѧ��ˮ�������������Һ���Ա�ʵ�飬�а�ɫ�����������÷�Ӧ�Ļ�ѧ����ʽ��AgNO3+NaCl=AgCl��+NaNO3����ˮ�����Ȼ��ƣ�
���������⿼��Ժ�ˮ�ĺ�������������������ˮ���Ǻ�ˮ����Ҫ����֮һ����ԭ����ʹ��ˮ������������ʹ�����������Ӷ��õ���ˮ�ķ�����

��ϰ��ϵ�д�
 ȫ��������ϵ�д�
ȫ��������ϵ�д�
�����Ŀ
 ������Դʮ�ַḻ��
������Դʮ�ַḻ����1����ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢���ˡ�
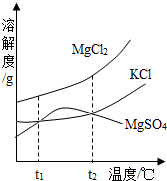
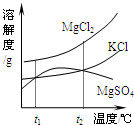
��2��ɹ�κ�õ���±ˮ�к���MgCl2��KCl��MgSO4�����ʣ���ͼ�����ǵ��ܽ������ʾ��ͼ��
��t1��ʱMgCl2��KCl��MgSO4�������ʵ��ܽ�ȷֱ�Ϊa��b��c�������ǵĴ�С��ϵΪ
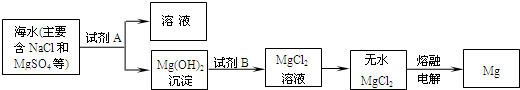
��3��Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�

����ȡMg�Ĺ����У��Լ�A����ѡ��
��þ����Ҫ�Ľ������ϣ��㷺Ӧ���ںϽ𡢻���ͷɻ�����ҵ�������ϴ�þ�ǴӺ�ˮ����ȡ�ģ�ijУѧϰС���ͬѧ�Լ��總����ˮ��þԪ�صĺ������вⶨ����ȡ��ˮ100kg��Ȼ���ټ����������Լ�A�����ˡ�ϴ�ӡ����������õ������������±����ظ�ʵ�����Σ���
| ʵ����� | ��һ��ʵ�� | �ڶ���ʵ�� | ������ʵ�� |
| ��ȡ��ˮ����/kg | 100 | 100 | 100 |
| ���ɳ�������/kg | 0.28 | 0.29 | 0.30 |
�۷����Mg��OH��2���NaCl��Һ�л���������CaCl2��Na2SO4��Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������BaCl2��Һ��Na2CO3��Һ�����ˣ�������Һ�м����������ᣮʵ���м������BaCl2��Һ��Ϊ�˳�ȥ
��4��Ŀǰ��ˮ�����ձ���á��༶����������֤������õ���ˮΪ��ˮ�ķ�����
 ���б��ٲ������нϳ��ĺ����ߣ�������Դʮ�ַḻ��
���б��ٲ������нϳ��ĺ����ߣ�������Դʮ�ַḻ��