��Ŀ����
����̼����"������ᴩ�������Ľ����У�
(1)����̼���С��Ľ��������________(�ѧʽ)������ŷţ��ܼ��������ů�����ʣ�
(2)�����������ж�����̼����������������Ҫԭ����________����Ȼ�������Ķ�����̼����Ҫ;����________��
(3)������ѧ��PaulSabatier���á����ת��"����ʹCO2��H2�ڴ�������������CH4��H2O����д���÷�Ӧ�Ļ�ѧ����ʽ________��
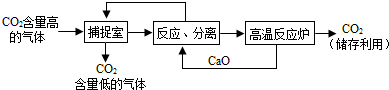
(4)����д��һ���ճ������з��ϡ���̼����"���������________��
�𰸣�
������
������
|
����(1)CO2 ����(2)ú�Ȼ�ʯȼ�ϵ�ȼ��ֲ��Ĺ������ ����(3)CO2��4H2����cH4��2H2O ����(4)�ᳫ�����г������С��˹����ȣ�һ(�����𰸺���������) |

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ