��Ŀ����
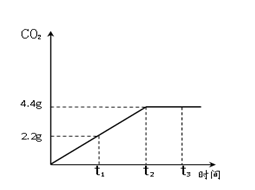
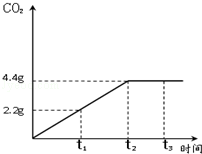
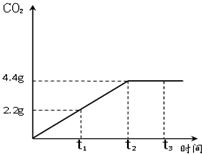
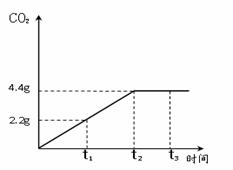
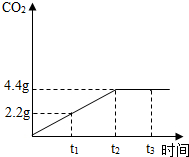 ʯ��ʯ�ǰ�ɫ�����ḻ�Ŀ����Դ֮һ��ij��ȤС��Ϊ�ⶨ����ʯ��ʯ����Ҫ�ɷ�CaCO3��������������ȡ12.5g��ʯ��ʯ��Ʒ���������ձ��У�����������ϡ���ᣬ��Ӧ����CO2��������ʱ���ϵ��ͼ�����裺ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ ����
ʯ��ʯ�ǰ�ɫ�����ḻ�Ŀ����Դ֮һ��ij��ȤС��Ϊ�ⶨ����ʯ��ʯ����Ҫ�ɷ�CaCO3��������������ȡ12.5g��ʯ��ʯ��Ʒ���������ձ��У�����������ϡ���ᣬ��Ӧ����CO2��������ʱ���ϵ��ͼ�����裺ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ ����������ϸ����۲�ͼ������ݣ��������������������⣺
��1����Ӧʱ��ﵽ
t2
t2
ʱ��̼���������ǡ����ȫ��Ӧ����2����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��������1������ͼ���ж�����̼�����ﵽ���ֵʱ��ʱ�������жϣ�
��2������������̼������������صĻ�ѧ��Ӧ����ʽ����̼��Ƶ���������������ʯ��ʯ��̼��Ƶ�����������
��2������������̼������������صĻ�ѧ��Ӧ����ʽ����̼��Ƶ���������������ʯ��ʯ��̼��Ƶ�����������
����⣺��1����ͼ���֪����Ӧ���е�t2ʱ���ɵĶ�����̼����Ϊ���ֵ����ʱ����̼��ƺ������ǡ�÷�Ӧ�㣻
��2����12.5g��Ʒ�к��е�̼���������x
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 4.4g
=
x=10g
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ
��100%=80%��
�ʴ�Ϊ��1��t2����2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ80%��
��2����12.5g��Ʒ�к��е�̼���������x
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 4.4g
| 100 |
| x |
| 44 |
| 4.4g |
x=10g
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ
| 10g |
| 12.5g |
�ʴ�Ϊ��1��t2����2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ80%��
���������⿼��ѧ�����û�ѧ��Ӧ����ʽ�ļ��㣬��ȷͼ������岢���ö�����̼���������м����ǽ��Ĺؼ���

��ϰ��ϵ�д�
 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�����Ŀ