��Ŀ����
������ij�������ļӸ�ʳ�ΰ�װ��ǩ�ϵIJ������֡�����ϸ�Ķ���ش��������⡣
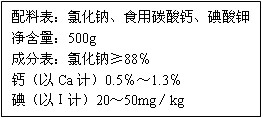
(1)��װ��ǩ�ϵĸƺ�����ָ________������ʸơ�����̼��ơ�������Ԫ�ء�����
(2)Ϊ�˼���������Ƿ���̼��ƣ��ڼ�ͥ�������ѡ�õ�������_________��
(3)Ϊ�˲ⶨ�����и�Ԫ�غ�����ȡ10g����������ˮ�������������ᣬ����0.132g������̼�������˼Ӹ�ʳ���и�Ԫ�ص������������������õ������ԭ��������O-16��C-12��Ca-40��H-1��Cl-35.5��
(2)Ϊ�˼���������Ƿ���̼��ƣ��ڼ�ͥ�������ѡ�õ�������_________��
(3)Ϊ�˲ⶨ�����и�Ԫ�غ�����ȡ10g����������ˮ�������������ᣬ����0.132g������̼�������˼Ӹ�ʳ���и�Ԫ�ص������������������õ������ԭ��������O-16��C-12��Ca-40��H-1��Cl-35.5��
(1)��Ԫ��
(2)ʳ��
(3)1.2%
(2)ʳ��
(3)1.2%

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ������ij�������ļӸ�ʳ�ΰ�װ��ǩ�ϵIJ������֣�����ϸ�Ķ���ش��������⣮
������ij�������ļӸ�ʳ�ΰ�װ��ǩ�ϵIJ������֣�����ϸ�Ķ���ش��������⣮


