��Ŀ����
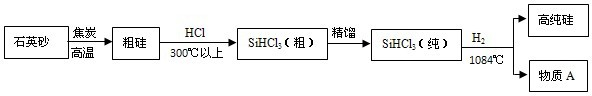
̫���ܹ��������ؼ��IJ����Ǹߴ��衣���ԭ�ӽṹ��ͼΪ�� ��������ͨ�����¹�����ȡ�ģ���һ���ƴֹ裺
��������ͨ�����¹�����ȡ�ģ���һ���ƴֹ裺
�������ƴ��裺��Si���֣�+2Cl2 SiCl4����SiCl4+2H2
SiCl4����SiCl4+2H2 Si������+4HCl
Si������+4HCl
��������ɫ���Ĺ�������SiO2���������ͨ����˵��ˮ������Ҳ���㲻��Ϥ����˵��ɳ�ӣ���һ������İ����ɳ�ӵ���Ҫ�ɷݼ���SiO2��SiO2�Ļ�ѧ������CO2��Щ���ơ������������Ϣ���ش��������⣺
��1��X��ֵΪ____________��
��2���ƶ�SiO2���������ʣ����㼴�ɣ�____________��___________��_______________��
��3����д��SiO2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��______________________��
��4���ڷ�Ӧ�٢ڢ��У�������_________��Ӧ���ͣ������û���Ӧ���ǣ�����ţ�_________��
��5����ѧ�Ͻ�ֻҪ��Ԫ�صĻ��ϼ۷����˱仯�Ļ�ѧ��Ӧ�ͽ���������ԭ��Ӧ��������ԭ��Ӧ�к��л��ϼ������˵�Ԫ�صķ�Ӧ��л�ԭ������Ӧ���еĻ�ԭ���ǣ�д��ѧʽ��__________��
 ��������ͨ�����¹�����ȡ�ģ���һ���ƴֹ裺
��������ͨ�����¹�����ȡ�ģ���һ���ƴֹ裺
�������ƴ��裺��Si���֣�+2Cl2
 SiCl4����SiCl4+2H2
SiCl4����SiCl4+2H2 Si������+4HCl
Si������+4HCl��������ɫ���Ĺ�������SiO2���������ͨ����˵��ˮ������Ҳ���㲻��Ϥ����˵��ɳ�ӣ���һ������İ����ɳ�ӵ���Ҫ�ɷݼ���SiO2��SiO2�Ļ�ѧ������CO2��Щ���ơ������������Ϣ���ش��������⣺
��1��X��ֵΪ____________��
��2���ƶ�SiO2���������ʣ����㼴�ɣ�____________��___________��_______________��
��3����д��SiO2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��______________________��
��4���ڷ�Ӧ�٢ڢ��У�������_________��Ӧ���ͣ������û���Ӧ���ǣ�����ţ�_________��
��5����ѧ�Ͻ�ֻҪ��Ԫ�صĻ��ϼ۷����˱仯�Ļ�ѧ��Ӧ�ͽ���������ԭ��Ӧ��������ԭ��Ӧ�к��л��ϼ������˵�Ԫ�صķ�Ӧ��л�ԭ������Ӧ���еĻ�ԭ���ǣ�д��ѧʽ��__________��
��1��14
��2����ɫ����������ˮ��Ӳ�ȴ��۵�ߡ����壨�������㣩
��3��SiO2+2NaOH==Na2SiO3+H2O
��4�����Ϸ�Ӧ���٢�
��5��C
��2����ɫ����������ˮ��Ӳ�ȴ��۵�ߡ����壨�������㣩
��3��SiO2+2NaOH==Na2SiO3+H2O
��4�����Ϸ�Ӧ���٢�
��5��C

��ϰ��ϵ�д�
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ