��Ŀ����
��2005?��ͷ����ͼ���й������ת����ϵ��ʾ��ͼ����������ת����ϵ�ƶϣ�

��1��B��
��2��F��M��Ӧ�Ļ�ѧ����ʽΪ
��3��A������

��1��B��
H2O
H2O
��D��CaCO3
CaCO3
���ѧʽ�����ƾ��ɣ�����2��F��M��Ӧ�Ļ�ѧ����ʽΪ
Ca��0H��2+Na2CO3�TCaCO3��+2NaOH��Ca��OH��2+K2CO3�TCaCO3��+2KOH
Ca��0H��2+Na2CO3�TCaCO3��+2NaOH��Ca��OH��2+K2CO3�TCaCO3��+2KOH
����3��A������
CH4
CH4
��C2H5OH����������ȷ���ɣ�
C2H5OH����������ȷ���ɣ�
���ѧʽ�����ƾ��ɣ�������������Ϊ��ͼʽ�ƶ��⣬����ؼ�����ͻ�ƿڣ�A��������ȼ������B��C��˵��AΪ�����D�����·ֽ�����E��C����DΪ̼��ƣ�̼��Ʒֽ����������ƺͶ�����̼��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ����B Ϊˮ��EΪ�����ƣ�FΪ�������ƣ�MΪһ�ֿ����Ե�̼���Σ�Aȼ�����ɶ�����̼��ˮ�������Ϊ������Ҵ���
����⣺�ɿ�ͼ����Ϣ��֪��D�ڸ����·ֽ������������ʣ���DΪ̼��ƣ��ֽ����������ƺͶ�����̼��A��������ȼ�����ɶ������ʣ����ƶ�AΪ̼�⻯���BΪˮ��CΪ������̼��C��F��Ӧ����̼���D����FΪ�������ƣ�BΪˮ����A�����Ǽ�����Ҵ���EΪ�����ƣ���Bˮ���������������ƣ�����������̼������Һ��Ӧ����̼��Ƴ�����
�ʴ�Ϊ����1��H2O��CaCO3��
��2��Ca��0H��2+Na2CO3�TCaCO3��+2NaOH��Ca��OH��2+K2CO3�TCaCO3��+2KOH��
��3��CH4 C2H5OH����������ȷ���ɣ�
�ʴ�Ϊ����1��H2O��CaCO3��
��2��Ca��0H��2+Na2CO3�TCaCO3��+2NaOH��Ca��OH��2+K2CO3�TCaCO3��+2KOH��
��3��CH4 C2H5OH����������ȷ���ɣ�
����������Ϊ��ͼʽ�ƶ��⣬����ؼ�����ͻ�ƿڣ�Ȼ��˳�˻����ƻ����м��Ƶķ�����ɣ�

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
 ��2005?��ͷ���ס������ֹ������ʵ��ܽ��������ͼ��ʾ���ֽ���֧�ֱ�װ�мס����������ʱ�����Һ���ײ�����δ�ܽ�Ĺ��壩���Թܽ���ʢ��ˮ���ձ��Ȼ�����ձ��м���һ������Ũ���ᣮ���Թ��ڲ�����������
��2005?��ͷ���ס������ֹ������ʵ��ܽ��������ͼ��ʾ���ֽ���֧�ֱ�װ�мס����������ʱ�����Һ���ײ�����δ�ܽ�Ĺ��壩���Թܽ���ʢ��ˮ���ձ��Ȼ�����ձ��м���һ������Ũ���ᣮ���Թ��ڲ�����������

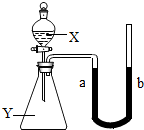 ��2005?��ͷ����ͼ��ʾ����ƿ��ʢ������Y������Ϊ���塢��Һ����壩����Һ©����ʢ��Һ��X��U�ι���ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ���ش��������⣺
��2005?��ͷ����ͼ��ʾ����ƿ��ʢ������Y������Ϊ���塢��Һ����壩����Һ©����ʢ��Һ��X��U�ι���ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ���ش��������⣺