��Ŀ����
��2012?������ģ��ijͬѧΪ�˲ⶨ��ͭм����п��ͭ�γɵĺϽ���Ʒ��ɣ�ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±����Լ��㣺
| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ������g�� | 50.0 | 50.0 | 50.0 | 50.0 |
| ȡϡ����������g�� | 40.0 | 80.0 | 120.0 | 160.0 |
| ��������������g�� | 0.4 | 0.8 | 1.0 | 1.0 |
��2����ʽ�����ͭм��Ʒ�е�п����������������ϡ���������ʵ�����������______��
���𰸡���������1�����ݵ�2����Ʒ�ķ�Ӧ���ݿ�֪������80gϡ����ʱ�����������������ӣ�˵����1����Ʒ��40gϡ���Ტδ����ȫ��Ӧ��������Ʒ��ʣ�࣬�������������ȫ��Ӧ��
��2�����ݵ�4����Ʒ�������ݿ�֪������1g����ʱ��Ʒ��п����ȫ��Ӧ���ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɲ�����������������Ʒ��п���������Ӷ��������Ʒ��п��������������1��2��3����Ʒ��ʹ����ϡ������ȫ��Ӧ����˿ɸ��������ε������������ϡ���������ʵ����������ļ��㣮
����⣺��1�����ڼ�������ϡ���������ʱ��������������Ҳ�����ӣ����жϵ�1����Ʒ���������ϡ������ȫ��Ӧ������Ʒ�е�п��ʣ�ࣻ
�ʴ�Ϊ�����
��2����μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 2
x 1.0g
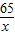 =
=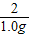 x=32.5g
x=32.5g
���ͭм��Ʒ�е�п����������=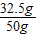 ×100%=65%
×100%=65%
���1����Ʒ��Ӧ�����������Ϊy
Zn+H2SO4�TZnSO4+H2��
98 2
y 0.4
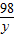 =
=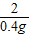 y=19.6g
y=19.6g
������ϡ���������ʵ���������=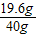 ×100%=49%
×100%=49%
�ʴ�Ϊ��65%��49%��
�����������ʱ��Ҫע������ϡ������������������ļ��㣬����ʱҪѡȡϡ������ȫ��Ӧʱ�����ݽ��м��㣮
��2�����ݵ�4����Ʒ�������ݿ�֪������1g����ʱ��Ʒ��п����ȫ��Ӧ���ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɲ�����������������Ʒ��п���������Ӷ��������Ʒ��п��������������1��2��3����Ʒ��ʹ����ϡ������ȫ��Ӧ����˿ɸ��������ε������������ϡ���������ʵ����������ļ��㣮
����⣺��1�����ڼ�������ϡ���������ʱ��������������Ҳ�����ӣ����жϵ�1����Ʒ���������ϡ������ȫ��Ӧ������Ʒ�е�п��ʣ�ࣻ
�ʴ�Ϊ�����
��2����μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 2
x 1.0g
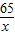 =
=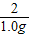 x=32.5g
x=32.5g���ͭм��Ʒ�е�п����������=
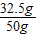 ×100%=65%
×100%=65%���1����Ʒ��Ӧ�����������Ϊy
Zn+H2SO4�TZnSO4+H2��
98 2
y 0.4
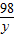 =
=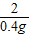 y=19.6g
y=19.6g������ϡ���������ʵ���������=
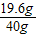 ×100%=49%
×100%=49%�ʴ�Ϊ��65%��49%��
�����������ʱ��Ҫע������ϡ������������������ļ��㣬����ʱҪѡȡϡ������ȫ��Ӧʱ�����ݽ��м��㣮

��ϰ��ϵ�д�
�����Ŀ