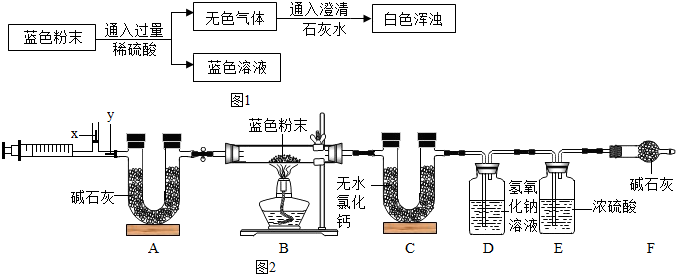
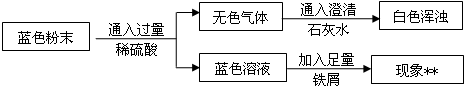
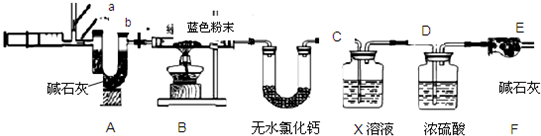
��Ŀ����
 ��������Na2CO3��Һ���뵽һ����CuSO4��Һ�еõ���ɫ���壮ij��ѧ��ȤС�����ɫ����ijɷֽ����˶���̽������֪��ΪCu��OH��2��CuCO3�Ļ���
��������Na2CO3��Һ���뵽һ����CuSO4��Һ�еõ���ɫ���壮ij��ѧ��ȤС�����ɫ����ijɷֽ����˶���̽������֪��ΪCu��OH��2��CuCO3�Ļ�����1����ɫ�����к���CuCO3��ԭ���ǣ�
��2����֪��Cu��OH��2�ķֽ��¶�Ϊ66�桫68�棬CuCO3�ķֽ��¶�Ϊ200�桫220�棬�Ҹ����ɶ�Ӧ���������������ɫ��������ΪaCu��OH��2?bCuCO3����С��ͬѧ���ȷ����ǶԹ�������ȷֽ⣬���������ݣ���ɹ��������仯��ֽ��¶ȵĹ�ϵ��ͼ��
��д��CD�η�����Ӧ�Ļ�ѧ����ʽ��
�ڸ�����ͼ�����a��b��ֵ����д��������̣�����������������ȱ�ʾ��
��D���Ӧ�������ֵΪ
��������1������̼����������ͭ��Ӧ����ʽ��д�����ǣ�����̼������Һ������Կ��ǣ�
��2���ٸ��ݼ���ˮҪ���ڼ��������̼��ǰ����з�����
�ڸ���������ͭ��̼��ͭ�ķֽ��¶Ƚ��з���������������ͭ��̼��ͭ�ֽ�Ļ�ѧ����ʽ���з�����
��2���ٸ��ݼ���ˮҪ���ڼ��������̼��ǰ����з�����
�ڸ���������ͭ��̼��ͭ�ķֽ��¶Ƚ��з���������������ͭ��̼��ͭ�ֽ�Ļ�ѧ����ʽ���з�����
����⣺��1��̼����������ͭ��Ӧ���ϸ��ֽⷴӦ��������Ӧ����̼����������ͭ����������̼��ͭ�������ƣ����Է���ʽ�ǣ�CuSO4+Na2CO3�TCuCO3��+Na2SO4��̼�����׳ƴ����Լ��ԣ�
��2����AB�ε��¶ȴ���60�浫��û�г���200�棬��ͼ���п���Ҳ������100�棬����������ͭ�ķֽ��¶ȣ�CD�ε��¶ȳ�����200�棬����̼��ͭ�ķֽ��¶ȣ����Է���ʽ�ǣ�CuCO3
CuO+CO2����
�ڽ⣺����ͼ�ɵ�AB����Cu��OH��2���ȷֽ⣬���ɵ�ˮ������Ϊ32.0g-28.4g=3.6g
������3.6gˮ�����Cu��OH��2������Ϊx��
Cu��OH��2
CuO+H2O
98 80 18
x Z 3.6g
����
=
��� x=19.6g
����CuCO3������Ϊ32.0g-19.6g=12.4g
������ɫ������ɿɵ�
=
���a��b=2��1
�����ϱߵĽ�����֪̼��ͭ����Ϊ12.4g������ȫ�ֽ�õ�����ͭ����ΪY��
CuCO3
CuO+CO2����
124 80
12.4g Y
���ݣ�
=
���̼��ͭ�ֽ�õ�����ͭ����Ϊ8g�����ݣ�
=
���Z=16g������D���Ӧ�������ֵΪ8g+16g=24g
�ʴ�Ϊ����1��CuSO4+Na2CO3�TCuCO3��+Na2SO4�� ���2����CuCO3
CuO+CO2��
��a��b=2��1
��24g
��2����AB�ε��¶ȴ���60�浫��û�г���200�棬��ͼ���п���Ҳ������100�棬����������ͭ�ķֽ��¶ȣ�CD�ε��¶ȳ�����200�棬����̼��ͭ�ķֽ��¶ȣ����Է���ʽ�ǣ�CuCO3
| ||
�ڽ⣺����ͼ�ɵ�AB����Cu��OH��2���ȷֽ⣬���ɵ�ˮ������Ϊ32.0g-28.4g=3.6g
������3.6gˮ�����Cu��OH��2������Ϊx��
Cu��OH��2
| ||
98 80 18
x Z 3.6g
����
| 98 |
| 18 |
| x |
| 3.6g |
����CuCO3������Ϊ32.0g-19.6g=12.4g
������ɫ������ɿɵ�
| 98a |
| 124b |
| 19.6g |
| 12.4g |
�����ϱߵĽ�����֪̼��ͭ����Ϊ12.4g������ȫ�ֽ�õ�����ͭ����ΪY��
CuCO3
| ||
124 80
12.4g Y
���ݣ�
| 124 |
| 80 |
| 12.4g |
| Y |
| 80 |
| 18 |
| Z |
| 3.6g |
�ʴ�Ϊ����1��CuSO4+Na2CO3�TCuCO3��+Na2SO4�� ���2����CuCO3
| ||
��a��b=2��1
��24g
�������ڽ������ʱ������Ҫ�����е�֪ʶ��֪��Ȼ����ѧ����֪ʶ���н�𣬴������ѶȽϴ�Ҫϸ�Ľ��з������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ