��Ŀ����
ij��ѧ��ȤС��ͨ���������ϵ�֪������������ȿɷֽ��仯ѧ����ʽΪ H2C2O4?2H2O CO��+CO2��+3H2O���ÿ���С���ͬѧ��ʵ���ҷֱ����������ʵ�飬��ش��������⣺
CO��+CO2��+3H2O���ÿ���С���ͬѧ��ʵ���ҷֱ����������ʵ�飬��ش��������⣺
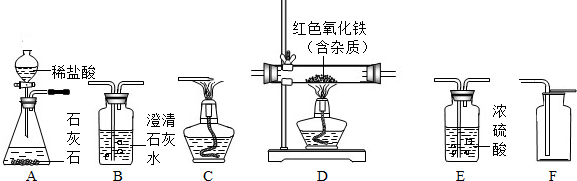
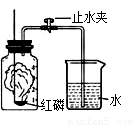
��1����ͬѧ����ͼ1װ����ȡ���ռ�CO��
��Bװ�õ����� ��
��CO����������ƿ��ˮ���мӸ�ȡ����Ϊ��ֹ����������Ӧ�� ��ѡ�a���Ȱѵ��ܴ�ˮ�����Ƴ�����b���Ͽ�A��B֮������ӡ���
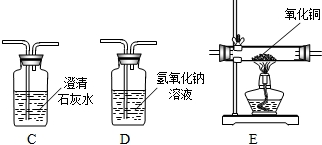
��2����ͬѧΪ����֤����ķֽ���������ĸ��������ֱ�����壨����ˮ����������ͨ�������������Լ���
A��ʯ��ˮ�����ȵ�����ͭ����ˮ����ͭ������������Һ
B����ˮ����ͭ������������Һ�����ȵ�����ͭ��ʯ��ˮ
C��ʯ��ˮ����ˮ����ͭ�����ȵ�����ͭ��ʯ��ˮ
D����ˮ����ͭ��ʯ��ˮ������������Һ�����ȵ�����ͭ��ʯ��ˮ
����Ϊ������ȷ�ķ����� ��
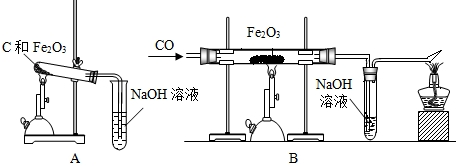
��3����ͬѧ��ͼ2װ�����ò���ֽ������CO�ⶨij���������������ɣ�
����Ʒ��������23.2g����Cװ��������������ȫ������ԭ����ʣ�������Ϊ16.8g���������������Ļ�ѧʽΪ ��
�ڶ�ͬѧ��Ϊȥ��Aװ�ã��Բⶨ����������Ļ�ѧʽ��� Ӱ�죨��С����ޡ���
��2����������Ʒ������Լ�����֤��������е����ã������ȷ��˳��Ի�������ÿһ�ɷֽ�����֤��
��3���ٸ��ݶ�����װ�������ķ�����Cװ�ü��ٵ�������������������Ԫ�ص�������������������������������Ԫ������Ԫ�ص�������������������������Ԫ�غ���Ԫ�ص����������������Ļ�ѧʽ��
�ڷ���Ϊ�ⶨ�������������ɸ�װ�õ����ã��Ը�װ�ý������ۣ����ж϶Բⶨ�����������Ӱ�죮
����⣺��1������������������Һ���շֽ�����еĶ�����̼���壬�Ӷ��ռ�����Ϊ������һ����̼��
���ռ���ϣ�Ϊ��ֹ����������Ӧ�Ȱѵ��ܴ�ˮ�����Ƴ����ٶϿ�A��B֮������ӣ�Ȼ��Ϩ��ƾ��ƣ�
��2�����������̼����Ҫ�ѻ������ͨ�����ʯ��ˮ�������ٳ���ʱ����ˮ������Ӱ����������ˮ�ļ��飬����ڼ��������̼��һ����̼����Ӧ����ʹ����ˮ����ͭ�����������е�ˮ��Ȼ�����ó���ʯ��ˮ���������̼��������ʹ���������̼��Ӧ���������������������Һ�����������̼���Ҫͨ������������Һ����������̼����ֹ��һ����̼��Ӧ����ĸ��ţ����ڼ���һ����̼ǰҪ�ȼ��������̼����ȫ���ն�����̼��������������ͨ����������ͭ�ͳ���ʯ��ˮ������һ����̼������ȷ����ӦѡD������
��3������ȫ��Ӧ��װ��C��ʣ���������16.8g����������������������������װ��C���ٵ�����Ϊ����������Ԫ�ص�������23.2g-16.8g=6.4g��
��������������ﻯѧʽΪFexOy������������Ԫ�غ���Ԫ�ص�������Ϊ��
 =
= ��������
�������� =
= �����Ը�����������Ļ�ѧʽ��Fe3O4��
�����Ը�����������Ļ�ѧʽ��Fe3O4����װ��A�dz�ȥ��������еĶ�����̼����Cװ�õ������仯����Ӱ�죬���ԶԲⶨ����������Ļ�ѧʽ�����Ӱ�죻
�ʴ�Ϊ��
��1���ٳ�ȥ������̼��CO2����
��a��
��2��D��
��3����Fe3O4��
���ޣ�
����������ʵ��Ŀ��ȷ����Ƶ�ʵ��װ�õ����ã��ǽ��ô�����Ļ�����ؼ��������������к���ˮ������ͨ�����ڵ�һ����

��8�֣�����������벻��������ijУ��ѧ��ȤС���ͬѧ����ʦ��ָ���������й�������ϵ��̽��ʵ�顣
̽��һ��ij��ѧ��ȤС���ͬѧ�Կ��������������IJⶨʵ�����̽����
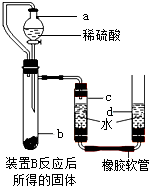
 ������ͼ��ʾװ�ú�ҩƷ����ʵ�飬��Ӧ�Ļ�ѧʽ��
������ͼ��ʾװ�ú�ҩƷ����ʵ�飬��Ӧ�Ļ�ѧʽ��
��ʽ�ǣ���������������������������������������
��С��ͬѧ��Ϊ������˿������ײⶨ������������
������С��ͬѧ��Ϊ�����ԣ���ԭ���ǣ���������
�� ����������������������������
��С��ͬѧ��ľ̿����������ⶨ�����������ĺ�����
���ȴ����ˮ����û�е�����С����ͬѧ������ϸ��
�飬����װ�õ������Լ���������������⡣����Ϊ
��ɴ������ԭ��������������������������������������
��С����ͬѧ��˼����ʵ���̽�����̺���Ϊ����ȼ�շ��ⶨ����������������ʵ��ʱ����ҩƷ��ѡ����������Ҫ����Ӧ���ǵ��ǣ� ��
̽�����������Ǹ�С��̽��Ӱ��H2O2��Һ��Ӧ���ʲ������ص����ʵ�����ݡ�
|
ʵ����� |
H2O2��ҺŨ��% |
H2O2��Һ���/mL |
�¶�/�� |
MnO2������/g |
�ռ����������/mL |
��Ӧ���� ��ʱ��/s |
|
�� |
5 |
1 |
20 |
0.1 |
4 |
16.75 |
|
�� |
15 |
1 |
20 |
0.1 |
4 |
6.04 |
|
�� |
30 |
5 |
35 |
0 |
2 |
49.21 |
|
�� |
30 |
5 |
55 |
0 |
2 |
10.76 |
��д��ʵ������H2O2��Һ��MnO2����������Ļ�ѧʽ����ʽ��_________________��
��ͨ��ʵ��ٺ͢ڶԱȿ�֪����ѧ��Ӧ������____________�йأ�
��ʵ��ۺܶ͢Աȿ�֪����ѧ��Ӧ�������¶ȵĹ�ϵ�ǣ�______________________��
��ͨ������ʵ��____����ܡ����ܡ���˵��ʹ��MnO2���Լӿ�H2O2��Һ��Ӧ���ʡ�