��Ŀ����
�ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㡣
��1����ˮ��ͭ���Ȼ������������У��ɷ��ӹ��ɵ��� ��
��2��ij�����ӵĽṹʾ��ͼΪ ����x����ֵ�����������е��� ��
����x����ֵ�����������е��� ��
A.9 B.10 C.11 D.12
��3��������A����������ȼ�ϡ���һ�������£���һ��������A��160g B����ͼ��ʾ��ַ�Ӧ����B��Ӧ��ȫʱ������132gC��72gD

�ٲμӷ�Ӧ��A���ʵ������� ��
����֪A����Է�������Ϊ44���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��5�֣�ÿ��1�֣���ѧ����ʽ2�֣�
��1��ˮ��2��CD��3�� 44g C3H8 + 5O2 = 3CO2 + 4H2O

��ϰ��ϵ�д�
�����Ŀ
�ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㣮����A��B��C��D�������ʣ�����ʾ��ͼ���±���
��1��1��B���ʷ������� ԭ�ӣ�
��2���������������ڵ��ʵ��� �������л������ ��[��д��ѧʽ]
��3��C����������Ԫ�ص�������Ϊ ��
��4����һ��������A��B��C��D�������ʷ���һ�ܱյ��������У��ڵ�������£�������ַ�Ӧ����÷�Ӧǰ������ʵ��������£�
���У�aֵΪ ���÷�Ӧ�Ļ�ѧ����ʽ��A��D�Ļ�ѧ������֮��Ϊ ��
| ���� | A | B | C | D |  --��ԭ�� --��ԭ�� --̼ԭ�� --̼ԭ�� --��ԭ�� --��ԭ�� |
| ��ʾ��ͼ |  |
 |
 |
 |
��2���������������ڵ��ʵ���
��3��C����������Ԫ�ص�������Ϊ
��4����һ��������A��B��C��D�������ʷ���һ�ܱյ��������У��ڵ�������£�������ַ�Ӧ����÷�Ӧǰ������ʵ��������£�
| A | B | C | D | |
| ��Ӧǰ����/g | 25 | 1 | 10 | 74 |
| ��Ӧ������/g | 9 | 37 | a | 10 |

 ������
������ ������
������ ���ֱ��ʾA��B��C�������ʵķ��ӣ���ͼ��ʾij��ѧ��Ӧǰ��Ӧ������������Ӽ�����Ŀ�ı仯���û�ѧ����ʽ��A��B��Cǰ�Ļ�ѧ������֮��Ϊ
���ֱ��ʾA��B��C�������ʵķ��ӣ���ͼ��ʾij��ѧ��Ӧǰ��Ӧ������������Ӽ�����Ŀ�ı仯���û�ѧ����ʽ��A��B��Cǰ�Ļ�ѧ������֮��Ϊ

 ��ʾAԪ�ص�ԭ�ӣ�
��ʾAԪ�ص�ԭ�ӣ� ��ʾBԪ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�
��ʾBԪ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�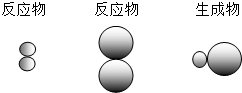
 ��
�� �е�
�е� ����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ��
����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ�� ��ʾ��Ԫ�ص�ԭ�ӣ�
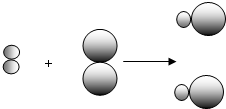
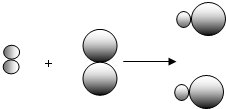
��ʾ��Ԫ�ص�ԭ�ӣ� ��ʾ��Ԫ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�
��ʾ��Ԫ�ص�ԭ�ӣ�ij��Ӧǰ���������ʾ��ͼ���£�


 ����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ��
����ͬ��Ԫ�أ�������Ϊ��Щԭ�Ӻ�����ͬ��




