��Ŀ����
6�֣���ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�顣Aװ�ü���ƿ��װ�������ԼΪ1:1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᡣ

��1���رջ���K1��K2����ע�����е���Һb����ƿ�У�����K1������B����Һ����A�к���Һ��Ϊ��ɫ��B�г���������Һ�档
��ش𣺢���a�����Ƕ�����̼����b�� ��Һ������b��ˮ��������a���е������� ��
��2������K1����״̬������K2��һ��ʱ���ر�K2�����������У��۲쵽D�е������� ��Cװ���з�Ӧ�Ļ�ѧ����ʽ�� ��
���𰸡�
��1������������ ��������ˮ����ˮ��Ӧ�����ɼ������ʣ�2�֣�
��2��D��Һ�����C�У�һ��ʱ�����©���е�Һ������
Fe2O3+3=Fe2(SO4)3+3H2O��1�֣� Fe + H2SO4=FeSO4+ H2����1�֣�
��������B����Һ����A�к���Һ��Ϊ��ɫ����֪A�е������Լ��ԣ����н��
��2������װ������ѹ��С���н��

��ϰ��ϵ�д�
�����Ŀ
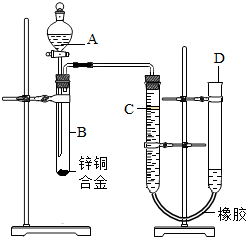 ��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������
��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������

