��Ŀ����
ijУ��ѧ�о���ѧϰС���ͬѧ��һƿ���ڷ��õ��������Ʋ�������Ȥ����������ͬѧΧ����ƿ�������ƽ�����һϵ�е�̽�����
�����������ƺ�̼���ƻ�����м�������ʱ�����������������Ʒ�Ӧ������̼���Ʒ�Ӧ��
��ȡ�����ù�����Ʒ�����Թ��У������м���ϡ���ᣬ���������ݲ�����˵������Ʒ�к���̼���ƣ��ɴ˿�ȷ���ù����ѷ������ʣ���Ӧ����ʽΪ��
��������������Ѿ���ȫ���ʣ�����ͬѧ��Ϊȡ������������ˮ�������еμ���ɫ��̪����ҺӦ����ɫ������ͬѧ˼������Ϊ����ͬѧ���뷨�Ǵ���ģ�ԭ���ǣ�
��
��Ϊ̽���ù������Ƿ���δ���ʵ��������ƣ�ͬѧ���ֽ��������±���ʾ��ʵ�飮�뽫�±���д������
ʵ������ⶨ����̼���Ƶ���������
�ټ���ͬѧ����ȡ20.00��Ʒ���������������ֱ����Ӧֹͣ�����ռ���4.40g������̼�������˼·��������Ʒ�����ᷴӦ���ɶ�����̼���������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�������������
������ͬѧ����ȡ20.00g��Ʒ����ˮ�����Һ������Һ�м�������ij���ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g�������˼·��������Ʒ��ʯ��ˮ��Ӧ���ɳ���̼��Ƶ������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�������������
��1���ڽ���ʱ����ʦ��ͬѧ�ǡ��ܷ�������ʵ�����õ����ݼ������Ʒ���������Ƶ����������������������ͬѧ��һ����Ϊ���ԣ���С��˼�����üס������ַ��������ף������ǣ�
��2��С������������·��������õ��ӳ�ȷ��ȡ20.00g���ʵ�NaOH��Ʒ������ƿ�У��õ��ӳӳӵ���ƿ����Ʒ��������Ϊ70.00g���ٰ�175.00g7.3%ϡ����ƽ���ֳ�7�����μ�����Ʒ�У�ÿ�γ�ַ�Ӧ�õ��ӳӳӵ���ƿ����ʢ���ʵ�������ʵ�����ݼ�¼���£�
��2�����������ʵ�����ݣ�������Ʒ���������Ƶ���������Ϊ
����Ҫд��������̣�
�������
�������
�����������Ƿ���ȫ���ʢ���Ʒ��̼���Ƶ����������Ƕ��٣���������
��������
�����������������̼��Ӧ����̼���ƺ�ˮ�������������ƺ�̼���ƻ�����м�������ʱ�����������������Ʒ�Ӧ������̼���Ʒ�Ӧ��
���ʵ��
���ʵ��
ʵ��һ��̽���������ƹ���ı����������ȡ�����ù�����Ʒ�����Թ��У������м���ϡ���ᣬ���������ݲ�����˵������Ʒ�к���̼���ƣ��ɴ˿�ȷ���ù����ѷ������ʣ���Ӧ����ʽΪ��
Na2CO3+2HCl=2NaCl+H2O+CO2��
Na2CO3+2HCl=2NaCl+H2O+CO2��
����������������Ѿ���ȫ���ʣ�����ͬѧ��Ϊȡ������������ˮ�������еμ���ɫ��̪����ҺӦ����ɫ������ͬѧ˼������Ϊ����ͬѧ���뷨�Ǵ���ģ�ԭ���ǣ�
̼���ƺ�����������Һ���Լ��ԣ�ʹ��̪��Һ��ɺ�ɫ
̼���ƺ�����������Һ���Լ��ԣ�ʹ��̪��Һ��ɺ�ɫ
��
��Ϊ̽���ù������Ƿ���δ���ʵ��������ƣ�ͬѧ���ֽ��������±���ʾ��ʵ�飮�뽫�±���д������
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| ��ȥ̼���� | ȡ�����ù�����Ʒ����ˮ�����Һ���μ��������Ȼ�����Һ����ַ�Ӧ����� | ������ɫ���� ������ɫ���� |
�йط�Ӧ�Ļ�ѧ����ʽΪ Na2CO3+CaCl2=2NaCl+CaCO3�� Na2CO3+CaCl2=2NaCl+CaCO3�� |
| �����Ƿ����������� | ����˺����Һ�е��� ��̪��Һ ��̪��Һ |
��� ��� |
����Ʒ�к����������� |
��Ʒ���
��Ʒ���
�ټ���ͬѧ����ȡ20.00��Ʒ���������������ֱ����Ӧֹͣ�����ռ���4.40g������̼�������˼·��������Ʒ�����ᷴӦ���ɶ�����̼���������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�������������
������ͬѧ����ȡ20.00g��Ʒ����ˮ�����Һ������Һ�м�������ij���ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g�������˼·��������Ʒ��ʯ��ˮ��Ӧ���ɳ���̼��Ƶ������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�������������
�������
�������
��������ͬѧ��ʵ����������������Ǽ������Ʒ��̼���Ƶ���������������ѡһ��д��������̣������뷴˼
�����뷴˼
��1���ڽ���ʱ����ʦ��ͬѧ�ǡ��ܷ�������ʵ�����õ����ݼ������Ʒ���������Ƶ����������������������ͬѧ��һ����Ϊ���ԣ���С��˼�����üס������ַ��������ף������ǣ�
��Ʒ����ˮ���������Ʒ���������Ƶ�����
��Ʒ����ˮ���������Ʒ���������Ƶ�����
����2��С������������·��������õ��ӳ�ȷ��ȡ20.00g���ʵ�NaOH��Ʒ������ƿ�У��õ��ӳӳӵ���ƿ����Ʒ��������Ϊ70.00g���ٰ�175.00g7.3%ϡ����ƽ���ֳ�7�����μ�����Ʒ�У�ÿ�γ�ַ�Ӧ�õ��ӳӳӵ���ƿ����ʢ���ʵ�������ʵ�����ݼ�¼���£�
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | ������ | ���ߴ� |
| ��ƿ����ʢ���ʵ������� | 95.00 | 120 | 145 | 170 | 192.8 | 215.6 | 240.6 |
40.0%
40.0%
����Ҫд��������̣�
������ʵ��һ���ٸ��������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
�ڸ���̼���ƺ�����������Һ�ļ��Է�����
�۸����Ȼ��ƺ�̼���Ʒ�Ӧ����̼��ư�ɫ�����Ȼ����Լ�����������ʹ��̪��Һ�����н��
ʵ�������������⡿���ݲ���������̼��������������̼��Ƶ��������̼���Ƶ��������������Ʒ��̼���Ƶ������������н��
�������뷴˼����1�������������ƾ�����ˮ�ԣ���Ʒ�г�ȥ̼���Ʋ�ֻ���������ƻ���ˮ���н��
��2�����������Ⱥ��������Ʒ�Ӧ��û���������ɣ����Կ�ʼ��Һ�������䣬�������Ʒ�Ӧ��Ϻ������ٺ�̼���Ʒ�Ӧ���ɶ�����̼���壬��������ٽ��н��
�ڸ���̼���ƺ�����������Һ�ļ��Է�����
�۸����Ȼ��ƺ�̼���Ʒ�Ӧ����̼��ư�ɫ�����Ȼ����Լ�����������ʹ��̪��Һ�����н��
ʵ�������������⡿���ݲ���������̼��������������̼��Ƶ��������̼���Ƶ��������������Ʒ��̼���Ƶ������������н��
�������뷴˼����1�������������ƾ�����ˮ�ԣ���Ʒ�г�ȥ̼���Ʋ�ֻ���������ƻ���ˮ���н��
��2�����������Ⱥ��������Ʒ�Ӧ��û���������ɣ����Կ�ʼ��Һ�������䣬�������Ʒ�Ӧ��Ϻ������ٺ�̼���Ʒ�Ӧ���ɶ�����̼���壬��������ٽ��н��
����⣺ʵ��һ���������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ����ʽΪNa2CO3+2HCl=2NaCl+H2O+CO2����
����Ϊ̼���ƺ�����������Һ���Լ��ԣ�ʹ��̪��Һ��ɺ�ɫ�����Բ������÷�̪��Һ���飻
���Ȼ��ƺ�̼���Ʒ�Ӧ����̼��ư�ɫ�������Ȼ��ƣ�����������Һ��ʹ��̪��Һ��죮
ʵ�������������⡿����̼��������Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 4.4g
=
x=10.6g
��Ʒ��̼���Ƶ���������=
��100%=53.0%
����Ʒ��̼���Ƶ���������53.0%��
�����̼��������Ϊy
Na2CO3+CaCl2=2NaCl+CaCO3��
106 100
y 10.00g
=
y=10.6g
��Ʒ��̼���Ƶ���������=
��100%=53.0%
����Ʒ��̼���Ƶ���������53.0%��
�������뷴˼����1���������ƾ�����ˮ�ԣ���Ʒ�г�ȥ̼���Ʋ�ֻ���������ƻ���ˮ���ʼס������ַ��������ף�
��2�������Ⱥ��������Ʒ�Ӧ��û���������ɣ����Կ�ʼ��Һ�������䣬�������Ʒ�Ӧ��Ϻ������ٺ�̼���Ʒ�Ӧ���ɶ�����̼���壬��������٣�
���������ݷ�����֪��ǰ�Ĵ���Һ����û�б仯������ǰ�Ĵη�Ӧ������������������ĵģ�
���������������������Ϊ175.00g��4/7=100.0g
����Ʒ���������Ƶ�����Ϊx��
NaOH+HCl=NaCl+H2O
40 36.5
x 100��Og��7.3%
=
x=8.0g
��Ʒ���������Ƶ���������=
��100%=40.0%
����Ʒ���������Ƶ���������Ϊ40.0%��
�ʴ�Ϊ��ʵ��һ����Na2CO3+2HCl=2NaCl+H2O+CO2����
�ڲ�����ɫ������Na2CO3+CaCl2=2NaCl+CaCO3���� ��̪��Һ����죻
ʵ���������̼��������Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 4.4g
=
x=10.6g
��Ʒ��̼���Ƶ���������=
��100%=53.0%
����Ʒ��̼���Ƶ���������53.0%��
�����̼��������Ϊy
Na2CO3+CaCl2=2NaCl+CaCO3��
106 100
y 10.00g
=
y=10.6g
��Ʒ��̼���Ƶ���������=
��100%=53.0%
����Ʒ��̼���Ƶ���������53.0%��
�������뷴˼����1�������ǣ���Ʒ����ˮ���������Ʒ���������Ƶ�������
��2��40.0%��
����Ϊ̼���ƺ�����������Һ���Լ��ԣ�ʹ��̪��Һ��ɺ�ɫ�����Բ������÷�̪��Һ���飻
���Ȼ��ƺ�̼���Ʒ�Ӧ����̼��ư�ɫ�������Ȼ��ƣ�����������Һ��ʹ��̪��Һ��죮
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| ��ȥ̼���� | ȡ�����ù�����Ʒ����ˮ�����Һ���μ��������Ȼ�����Һ�� ��ַ�Ӧ����� |
��ɫ���� | �йط�Ӧ�Ļ�ѧ����ʽΪ Na2CO3+CaCl2=2NaCl+CaCO3�� |
| �����Ƿ����������� | ����˺����Һ�е����̪��Һ | ��� | ����Ʒ�к����������� |
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 4.4g
| 106 |
| x |
| 44 |
| 4.4g |
��Ʒ��̼���Ƶ���������=
| 10.6g |
| 20g |
����Ʒ��̼���Ƶ���������53.0%��
�����̼��������Ϊy
Na2CO3+CaCl2=2NaCl+CaCO3��
106 100
y 10.00g
| 106 |
| y |
| 100 |
| 10.00g |
��Ʒ��̼���Ƶ���������=
| 10.6g |
| 20g |
����Ʒ��̼���Ƶ���������53.0%��
�������뷴˼����1���������ƾ�����ˮ�ԣ���Ʒ�г�ȥ̼���Ʋ�ֻ���������ƻ���ˮ���ʼס������ַ��������ף�
��2�������Ⱥ��������Ʒ�Ӧ��û���������ɣ����Կ�ʼ��Һ�������䣬�������Ʒ�Ӧ��Ϻ������ٺ�̼���Ʒ�Ӧ���ɶ�����̼���壬��������٣�
���������ݷ�����֪��ǰ�Ĵ���Һ����û�б仯������ǰ�Ĵη�Ӧ������������������ĵģ�
���������������������Ϊ175.00g��4/7=100.0g
����Ʒ���������Ƶ�����Ϊx��
NaOH+HCl=NaCl+H2O
40 36.5
x 100��Og��7.3%
| 40 |
| x |
| 36.5 |
| 100.0g��7.3% |
��Ʒ���������Ƶ���������=
| 8.0g |
| 20g |
����Ʒ���������Ƶ���������Ϊ40.0%��
�ʴ�Ϊ��ʵ��һ����Na2CO3+2HCl=2NaCl+H2O+CO2����
�ڲ�����ɫ������Na2CO3+CaCl2=2NaCl+CaCO3���� ��̪��Һ����죻
ʵ���������̼��������Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 4.4g
| 106 |
| x |
| 44 |
| 4.4g |
��Ʒ��̼���Ƶ���������=
| 10.6g |
| 20g |
����Ʒ��̼���Ƶ���������53.0%��
�����̼��������Ϊy
Na2CO3+CaCl2=2NaCl+CaCO3��
106 100
y 10.00g
| 106 |
| y |
| 100 |
| 10.00g |
��Ʒ��̼���Ƶ���������=
| 10.6g |
| 20g |
����Ʒ��̼���Ƶ���������53.0%��
�������뷴˼����1�������ǣ���Ʒ����ˮ���������Ʒ���������Ƶ�������
��2��40.0%��
�����������ؼ���Ҫ֪��̼�����Լ��ԣ�Ҳ��ʹ��̪��Һ���ɫ��ֻ���Ȱ�̼���Ƴ��������ټ����̪��Һ����֤�Ƿ����������ƣ��ٽ��м���ʱ���������غ���㷴Ӧ����Һ�����Ƚ�ֱ�ۣ���Ӧ�Ȳ�������������������Ӧ��������Һ������Ϊ��Ӧǰ����Һ�������ͣ�

��ϰ��ϵ�д�
�����Ŀ
Ϊ�˲ⶨijƷ��ʳ�ô�����̼���Ƶ�����������ijУ��ѧ�о���ѧϰС���̽���������£�
[�������]��Ʒ��̼���Ƶ����������Ƕ��٣�
[֪ʶ��]
ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
[��Ʒ�����ʵ��]
����ͬѧ����ȡ12.00��Ʒ����ˮ�����Һ������Һ�м����������ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g��
����ͬѧ����ȡ12.00��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���4.4g������̼��
[�������]
������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ������� ��̼���Ƶ����������� ������������ȷ��0.1%��
[������˼]
��1�������С��ͬѧ��Ϊ��Ҫ���̼���Ƶ�������Ҳ����ʹ���������ʯ��ˮ�������ͬ���������� ����һ�־������ʵĻ�ѧʽ������Һ����Ʒ��Ӧ��ͨ���ⶨ������ʵ������������йؼ��㼴�ɣ�
��2�������С��ͬѧ��Ϊ������ϡ�����������������Ҳ�������ȡ13.5g��Ʒ�����ձ��У�ÿ�μ���20gϡ���ᣨ������ˮ���Ȼ����ݳ������þ���������������¼ʵ���������£�
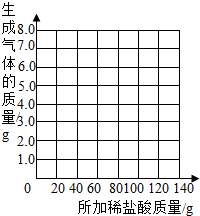
��������a= g��b= g�����������±ߵ�����ֽ�ϻ����������������������ϡ����������ϵ�����ߣ�
[�������]��Ʒ��̼���Ƶ����������Ƕ��٣�
[֪ʶ��]
ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
[��Ʒ�����ʵ��]
����ͬѧ����ȡ12.00��Ʒ����ˮ�����Һ������Һ�м����������ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g��
����ͬѧ����ȡ12.00��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���4.4g������̼��
[�������]
������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ�������
[������˼]
��1�������С��ͬѧ��Ϊ��Ҫ���̼���Ƶ�������Ҳ����ʹ���������ʯ��ˮ�������ͬ����������
��2�������С��ͬѧ��Ϊ������ϡ�����������������Ҳ�������ȡ13.5g��Ʒ�����ձ��У�ÿ�μ���20gϡ���ᣨ������ˮ���Ȼ����ݳ������þ���������������¼ʵ���������£�
| ��������Ĵ��� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| �ձ�����������������/g | 78.9 | 97.8 | 116.7 | 135.60 | 155.05 | 175.05 | 195.05 |
| �������������/g | 1.1 | 2.2 | a | 4.4 | 4.95 | b | -- |

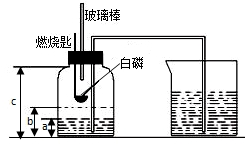 ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�
ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�