��Ŀ����
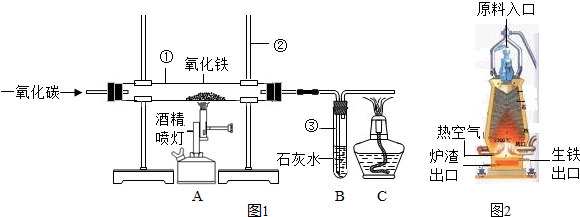
��ͼ1��ʵ����ģ��������װ��ͼ���Իش�

��1�������ٵ�������________�������ڵ�������________��
��2��ʵ������������з�����Ӧ�Ļ�ѧ����ʽ��__________���������е�������_________��
��3��ʵ������в�����β������ֱ���ŷŵ�ԭ����_________��
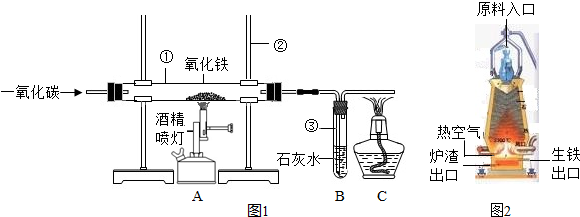
��4��ͼ2�ǹ�ҵ������������¯�Ľṹͼ��ʵ��������������ԭ������ʯ����̿��ʯ��ʯ�Ǵ�ԭ����ڼ��룬���н�̿�ڸ�¯����ʱ������֮һ�Dz������£���һ����Ϊ________���������ڵ���¯�����ڵ�ԭ����_____________��
��5��ȡ������¯�����������������ձ��У���������ϡ���ᣬ�ɹ۲쵽��������________��������Ӧ�Ļ�ѧ����ʽΪ___________������Ӧֹͣ���ɹ۲쵽�ձ��ײ��к�ɫ�������������Ҫ��________���ѧʽ����
��2��ʵ������������з�����Ӧ�Ļ�ѧ����ʽ��__________���������е�������_________��
��3��ʵ������в�����β������ֱ���ŷŵ�ԭ����_________��
��4��ͼ2�ǹ�ҵ������������¯�Ľṹͼ��ʵ��������������ԭ������ʯ����̿��ʯ��ʯ�Ǵ�ԭ����ڼ��룬���н�̿�ڸ�¯����ʱ������֮һ�Dz������£���һ����Ϊ________���������ڵ���¯�����ڵ�ԭ����_____________��
��5��ȡ������¯�����������������ձ��У���������ϡ���ᣬ�ɹ۲쵽��������________��������Ӧ�Ļ�ѧ����ʽΪ___________������Ӧֹͣ���ɹ۲쵽�ձ��ײ��к�ɫ�������������Ҫ��________���ѧʽ����
(1)�ٲ����� ������̨
(2)Fe2O3+3CO 2Fe+3CO2�������
2Fe+3CO2�������
(3)β���е�һ����̼��Ⱦ����
(4)��ԭ�����������ܶȴ���¯��
(5)�����������ݣ���Һ��Ϊdz��ɫ��Fe + 2HCl==FeCl2 + H2����C
(2)Fe2O3+3CO
 2Fe+3CO2�������
2Fe+3CO2�������(3)β���е�һ����̼��Ⱦ����
(4)��ԭ�����������ܶȴ���¯��
(5)�����������ݣ���Һ��Ϊdz��ɫ��Fe + 2HCl==FeCl2 + H2����C

��ϰ��ϵ�д�
�����Ŀ